COVID-19 Vaccine
Storage & Handling Checkup: Temperature Monitoring
Storage & Handling Checkup: Temperature Monitoring
- Digital Data Logger (DDL) – records temperatures, 24 hours a day, 7 days a week.
- Why? This provides documentation that every dose in your unit has been stored at the right temperature from the time you received it until it is administered to a patient.
- Paper Temperature Logs – records twice daily temperature checks.
- Why? This provides a chance to visually check the unit and ensure that the temperatures have not been out out range.
Limited Single Dose Vials of Pfizer Bivalent Becoming Available
Limited Single Dose Vials of Pfizer Bivalent Becoming Available
On Monday, November 14 through Tuesday, November 15, PDPH will open pre-ordering in Philavax for a limited amount of the updated (bivalent) Pfizer-BioNTech COVID-19 vaccine for people 12 years of age or older in single-dose vials. Deliveries will begin several days later. Single dose vials will not be available for ordering through Monday.com – partial ordering – or pickup.This limited rollout is intended to expand the number of locations that offer the updated vaccine. Only sites that would otherwise not be able to utilize the standard six-dose per vial bivalent Pfizer will be approved to order single dose vials. Large health systems and sites with regular patient volumes/throughput should continue to order the six-dose per vial bivalent Pfizer.Ancillary kits will not be distributed with orders of single-dose vials. Single-dose vials do not require the use of low-dead-volume (LDV) syringes.Quick Facts:Single-Dose Vial Pfizer Bivalent Booster (Ages 12+)• NDC: 59267-1404-02• Configuration: 10 single-dose vials/carton• Minimum quantity per order: 50 doses• Maximum quantity per order: 150 doses• Product dimensions: 1.457 in length × 1.535 in width × 3.504 in height• Storage and handling: Same as other Pfizer tris products (e.g., store at ultra-low temperature until expiry, may refrigerate up to 10 weeks within expiry period)• No ancillary kits included with single-dose vial orders
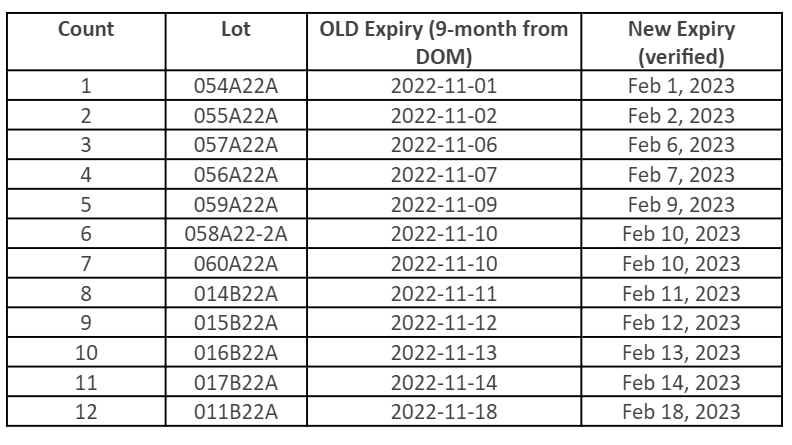
Moderna Shelf Life Extension
Moderna Shelf Life Extension
FDA has approved a shelf-life extension for the following Moderna10 products, which have been extended by three months. Below, find a table with updated expiration dates for the specific lots impacted by the extension. For the most current information, please visit the Moderna website.
Matchmaking Program Coming to an End
Matchmaking Program Coming to an End
The Philadelphia Department of Public Health’s COVID-19 Matchmaking Program would like to send a huge thank you for your time, commitment, and expertise you’ve given in partnering with vaccination clinics to provide vaccine. After careful consideration, we have determined to end the program effective November 4, 2022. 57 vaccination clinics were successfully matched for the duration of PDPH’s COVID-19 Matchmaking Program, and 77 providers were enrolled. These vaccinations clinics included community-based clinics, health fairs, and cultural events to name a few!If patients are looking to get vaccinated at their earliest convenience, you may direct them to use this link to find a facility in close proximity to them. Again, we thank you for your contributions and efforts to partner with vaccinations clinics to serve vaccine. You have made an immense difference through your dedication and continued support!
Temperature Reporting for Online Vaccine Ordering
Temperature Reporting for Online Vaccine Ordering
Temperature logs must be submitted on the same day as any order placed for federal vaccine (VFC, VFAAR, flu, COVID-19, or monkeypox)!
Temperature logs are to be uploaded in PhilaVax and emailed to tempcheck@phila.gov. The logs must show that the unit is within the appropriate temperature ranges and that there have been no alarms. This reporting guide can assist you in submitting your DDL temperature files.
For more information on ordering and how to upload your temperature log, please attend the next online ordering training on November 10, 2022 from 10:00AM – 11:00AM EST. Click the link below to register for the training.
If your site uses a privately purchased DDL or cannot upload in PhilaVax, please contact tempcheck@phila.gov.
Thank you for keeping Philadelphia safe and healthy!
Immunization Techniques Training
Immunization Techniques Training
Wednesday, October 12, 2022, from 8:30 am – 12:00 pm, 1:00 pm – 4:30 pm
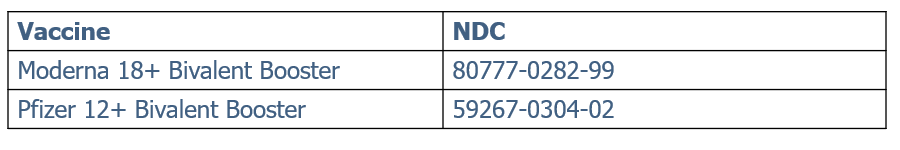
Moderna Bivalent Booster Ordering Resumed
Moderna Bivalent Booster Ordering Resumed
Moderna Bivalent Booster ordering has been turned back on in Philavax and Monday.com. Please note: allocations are still limited at this time so providers may not receive what is requested based on dose availability.
Please make sure you are selecting the correct NDC when ordering bivalent boosters (see table below). Selecting the monovalent product code and then selecting booster in the Dose Number column will not allow you to order bivalent doses. You will need to select the correct NDC. All previously submitted orders of bivalent vaccines during the ordering pause will need to be resubmitted for processing.