Minimum Ordering Quantity Reduction
Minimum Ordering Quantity Reduction
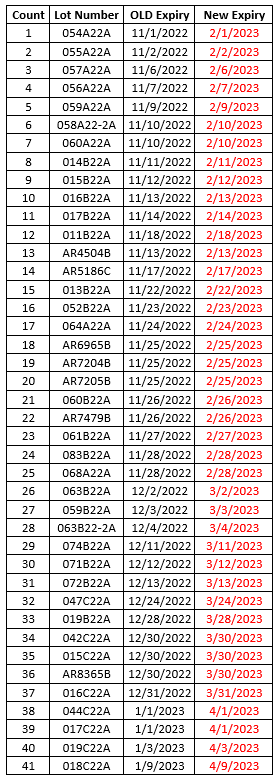
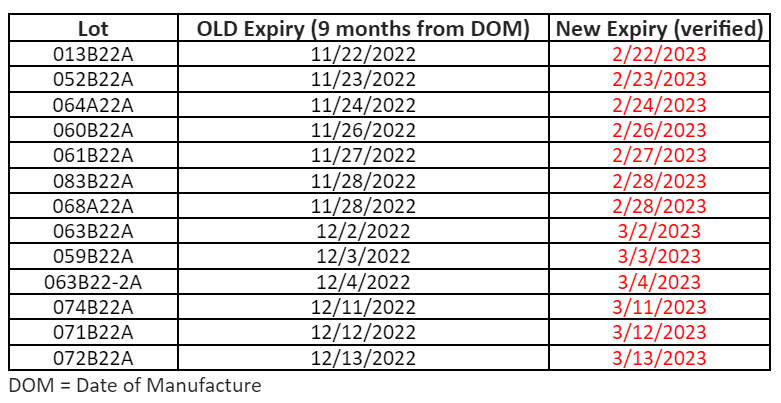
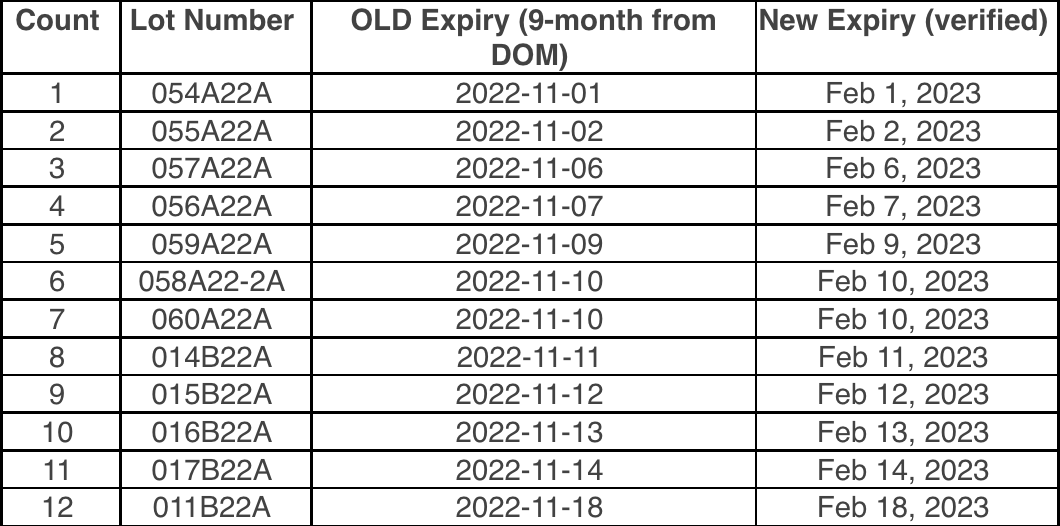
The minimum order quantity for bivalent Moderna 6mo – 5yr old (NDC 80777-0283-99) vaccine is being reduced to 20 doses per order. As a reminder, one carton of this vaccine contains 20 doses in 10 two-dose vials.Because ancillary supplies are packed well in advance of demand, they will continue to be shipped in minimum quantities to support 100 administrations per kit with this product. If you have sufficient supplies to support administration of the doses you order, we recommend opting out of receiving ancillary kits when placing orders for Moderna bivalent pediatric vaccines.