COVID-19 Vaccine
Bivalent Pfizer and Moderna Boosters Are Coming
Bivalent Pfizer and Moderna Boosters Are Coming
- Pre-ordering for both Pfizer and Moderna bivalent boosters will begin on Monday, August 22, 2022, and close Wednesday, August 24, 2022, at 5pm.
- Sites should plan to manage necessary freezer/refrigerator space as well as inventory/ordering to avoid waste when developing their overall fall vaccine plans.
- These bivalent formulas can only be used for boosters for individuals who have completed their primary series.
- Bivalent boosters will include a BA.4/5 valence specifically.
- The original Pfizer and Moderna vaccine formulas should only be used for primary series vaccinations once the bivalent products arrive.
- As the cap colors/borders will be the same for bivalent boosters and the original formulas, sites will need to be mindful of packaging/labeling to differentiate the two products.
- Delivery will begin after Labor Day – date TBD.
- ACIP will meet to make official recommendations about vaccine intervals and dosing schedules.
- EUAs for these two bivalent products are not yet available.
- It is expected that at least one bivalent vaccine for children ages 11 years and younger may be authorized within a short time following the authorization(s) of bivalent vaccines for people ages 12 years and older.
Full CDC Vaccination Record Cards
Full CDC Vaccination Record Cards
- Complete a new card for the patient
- Staple both cards together
- Encourage the patient to photograph both cards in case they become separated
- Bring both cards to future vaccination appointments
Partner to Hold Vaccine Clinics!
Partner to Hold Vaccine Clinics!
Dear COVID-19 Vaccine Providers,
PDPH is experiencing an uptick in community organizations requesting vaccine at events.
If you have not signed up already and are interested in being partnered with a community organization to provide them COVID-19 vaccine at events, please sign up using the link below.
Thank you to all providers that are currently signed-up to be matched with Philadelphia-based organizations who are seeking COVID-19 vaccine for their community members. If you have already filled out the survey, there is no need to fill it out again.
Fill out our vaccine provider capacity survey!
In the event that PDPH has an organization seeking a vaccine provider, you will be contacted by PDPH to confirm your interest in partnering to hold the clinic before any contact information is shared.
Thank you for keeping Philadelphia safe and healthy. If you have any questions, please email vaccines@phila.gov.
Novavax Vaccine Available for Limited Ordering
Novavax Vaccine Available for Limited Ordering
- Novavax is the first COVID-19 protein subunit vaccine that the CDC has recommended for use in the United States.
- ACIP’s vote—which follows the U.S. Food and Drug Administration (FDA)’s emergency use authorization—is for the use of the Novavax COVID-19 vaccine for adults ages 18 and older as a two-dose primary series.
- This recommendation is for all adults, including those who are moderately or severely immunocompromised.
- Individuals who receive Novavax are not eligible for any boosters at this time.
- Allocations of Novavax will be limited.
- Participating Federal Retail Pharmacies will be eligible to receive Novavax.
- Ordering for Novavax will open up on Monday 7/25/22.
- The minimum order quantity is 100 doses. Partial, Monday.com, ordering is not currently available.
- Presently, we are expected to receive only one allocation of Novavax vaccine for our providers to order against. As this initial allocation will be limited and jurisdictions will need to manage first and second doses with it, orders will be prioritized and restricted. Placing an order for Novavax does not guarantee a shipment.
- Novavax is a two-dose COVID vaccination series, given as a 0.5ml IM injection three weeks apart.
- It is stable at refrigeration only, currently for up to nine months.
- Initial shipments are set to expire sometime in February 2023.
- There will be a website available to search expiration dates by lot number.
- No expiration information will be printed on the vials
- Once punctured, the vial needs to be discarded within 6 hours.
Be Ready!: Update Your Vaccine Management Plan Today!
Update Your Vaccine Management Plan Today!
Summer means storm season, and to help you be prepared, we’ve created a new Vaccine Management Plan.
It has all the information you need to:
- Store your vaccine safely.
- Respond to an emergency.
- Transport your vaccine during an emergency.
Don’t wait until the storm, comes take action today:
- Review the document with all the staff that works with VFC, VFAAR, or COVID vaccine at your site.
- Gather the supplies indicated on pages 14-17 of the document.
- Fill out the information on the first page of the document.
- Fax (215-238-6948) or email (TempCheck@phila.gov) a copy to our office by Friday, August 12th.
- Post the full document on your vaccine storage unit.
- You can print a copy today or keep an eye out for a copy in the mail this week!
Make sure your site’s backup storage locations are appropriate for vaccine storage:
- Approved
- Doctor’s offices freezer compartment of combination units
- Hospital Pharmacies
- Pharmacies
- Approved Unit at Another Clinic
- Not Approved
- Personal Home Freezers
- Personal Deep Freezers
- Freezer Compartment of a Combination Unit
- Storage Containers with Dry Ice
Be ready to move vaccine in an emergency. Gather the supplies needed to transport your refrigerated and frozen vaccine today!
Have questions? Reach out to our team at TempCheck@phila.gov for assistance.
Vaccine Updates: Moderna Life Span Approval, Training and Resources & July 4th Deliveries
Vaccine Updates: Moderna Life Span Approval, Training and Resources & July 4th Deliveries
- Moderna 6-11 Years: A two-dose Moderna COVID-19 vaccines series (50ug) is recommended for children ages 6-11 years, under the EUA issued by FDA.
- Ordering for this product, Dark Blue Cap/Purple Border, will open next week in Philavax on the 29th. Monday.com ordering will open the following week.
- Moderna 12-17 Years: A two-dose Moderna COVID-19 vaccines series (100ug) is recommended for children ages 12-17 years, under the EUA issued by FDA.
Training and Resources
- Vial Wallchart – Moderna COVID-19 Vaccines (see also this version)
- Vial for Peds 6mths-5years – Moderna Peds COVID-19 Vaccines
- What to Expect – HCP Letter – Moderna Peds COVID-19 Vaccines
- Videos – Dosing and Administration Moderna Peds COVID-19 Vaccines
- Dear Healthcare Provider Letter – Moderna 6-11 COVID-19 Vaccine
- LOA – Moderna 6mths – 17yrs COVID Vaccines
- Tuesday, June 28 at 3 pm ET (2 pm CT / 12 pm PT)
- Thursday, July 7 at 12 pm ET (11 am CT / 9 am PT)
- Tuesday, July 12 at 2 pm ET (1 pm CT / 11 am PT)
- Tuesday, July 19 at 12 pm ET (11 am CT / 9 am PT)
- Trainings now include presentations on COVID-19 vaccines with a MAROON Cap for individuals 6 months through 4 years of age.
- For details, see dates and links for upcoming training sessions.
- Daily trainings (Monday through Friday) will begin Monday, June 20, 2022, and continue at least through July 8, 2022 (with no training on July 4th).
Fourth of July Deliveries
- No vaccine deliveries will occur Saturday July 2 through Tuesday, July 5, 2022.
- No vaccine deliveries will occur on Saturday, July 2 through Monday, July 4, 2022.
Storage Unit Reminder
Storage Unit Reminder
Vaccines can only be stored in certain acceptable storage units. Your vaccine storage unit is a key component in maintaining the vaccine cold chain at your practice.
Your vaccine storage units must:
- Consistently maintain storage temperature.
- Have enough space to properly store vaccines throughout the year including back to school and flu season.
- Have separate external doors for refrigerator and freezer.
- Have Do Not Unplug stickers at the outlet, on unit, and on the circuit breaker.
- Order Do Not Unplug stickers here.

All units must be plugged directly into a dedicated wall outlet. Units cannot be plugged into:
- GFI/GFCI outlets (specialized outlets with a built-in breaker, may have reset buttons)
- Outlets that can be activated by a wall switch
- Extension cords, power strips, surge protectors
- Ensure that the unit, plug, and circuit breaker on the electric panel are clearly labeled to prevent accidental loss of power.
Selecting a unit to store your vaccines in is an important decision.
Purchasing a unit that is reliable and accurate up-front is a worthwhile investment, both in time and money, for your practice. The time needed to respond to out of range temperatures and the cost of reimbursement for wasted vaccines can be very burdensome for practices whose unit does not maintain in-range temperatures.
To ensure that the unit that you are considering is the best option for your clinic, email our program at TempCheck@phila.gov so that we can offer insight on the units that are being considered based on other providers experiences with a brand, the typical supply on hand at your practice, and other considerations based on the CDC recommendations for storage units.
COVID-19 Vaccine Ordering Deadline
COVID-19 Vaccine Ordering Deadline
The Philadelphia Department of Public Health (PDPH) Immunization Program would like to remind COVID-19 providers that the deadline to order COVID-19 vaccine is Wednesday at 5pm for orders placed both through Philavax and Monday.com. Orders placed after the deadline will not be processed.
Vaccine orders are delivered on the following Tuesday or Wednesday; please plan accordingly for upcoming clinics.
Quick Steps to Order:
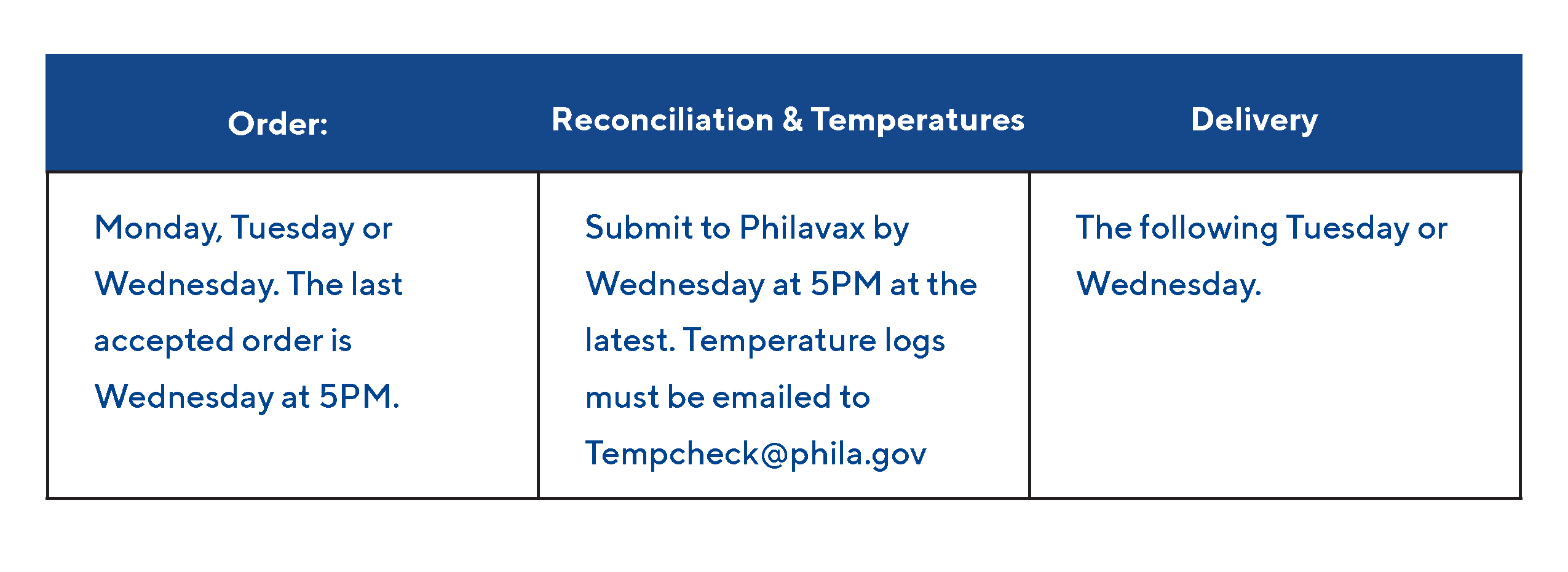 Going forward, the Immunization Program will not process any orders that do not have a closed reconciliation and submitted temperatures by Wednesday at 5PM.
Going forward, the Immunization Program will not process any orders that do not have a closed reconciliation and submitted temperatures by Wednesday at 5PM.
If you have questions, please reach out to vaccines@phila.gov.
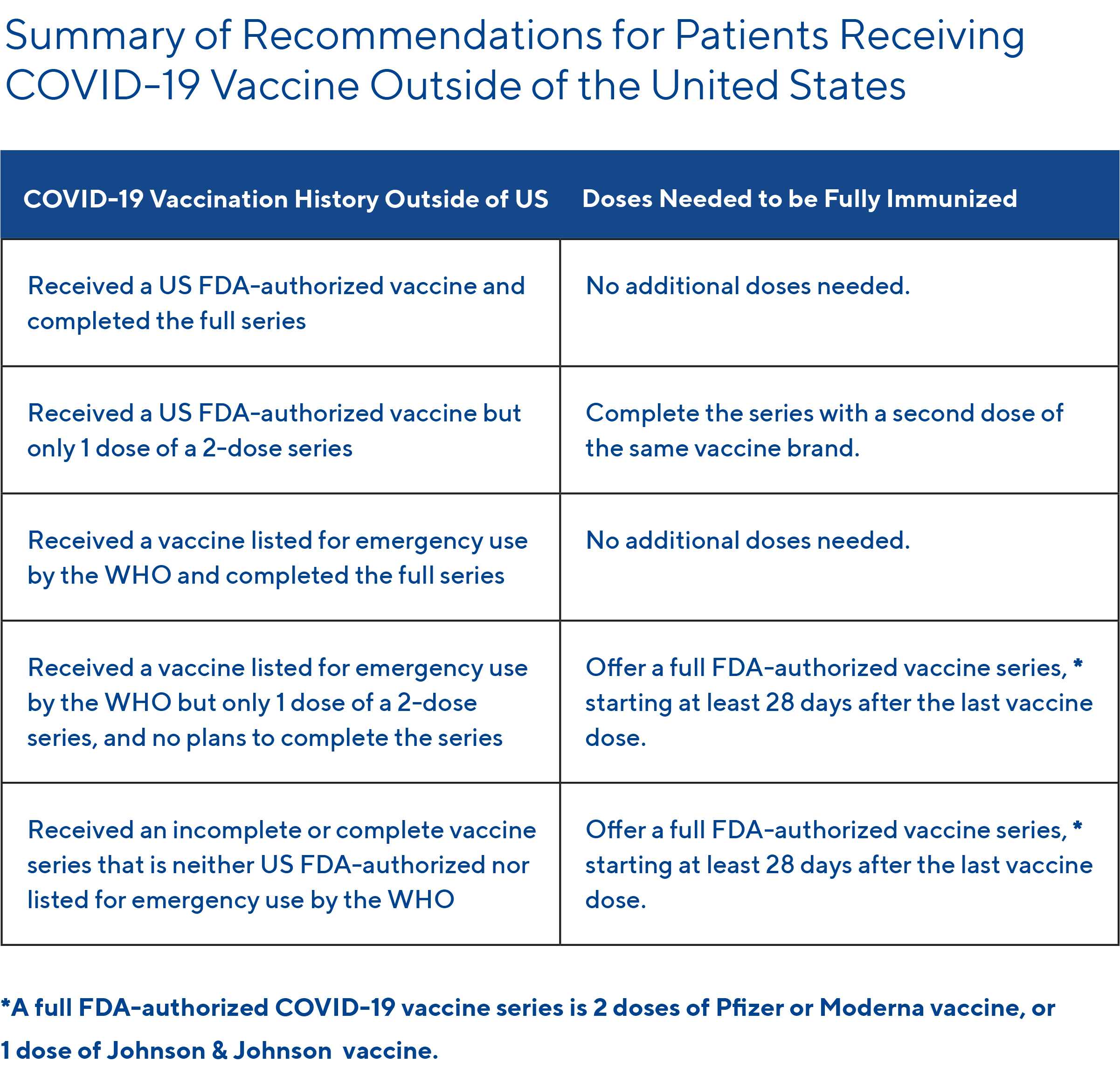
Did Your Patient Receive a COVID-19 Vaccination Outside the United States?
Did Your Patient Receive a COVID-19 Vaccination Outside the United States?
Have questions? The “People vaccinated outside the United States” section of the CDC’s Interim Clinical Considerations for Use of COVID-19 Vaccines provides guidance on different scenarios.
Talk to your patient and gather the initial information:
- Which COVID-19 vaccine did the patient receive?
- Is the COVID-19 vaccine authorized by the United States Food and Drug Administration (FDA) or listed for emergency use by the World Health Organization (WHO)?
- Did your patient complete a full series of this COVID-19 vaccine (e.g. receive both doses if two doses are recommended)?
Guidelines as of 7/15/21:
US FDA-authorized COVID-19 vaccines:
- Pfizer-BioNTech (Comirnaty, Tozinameran; mRNA nucleoside modified) – 2 doses
- Moderna (Spikevax; mRNA nucleoside modified) – 2 doses
- Johnson & Johnson / Janssen (Ad26.COV2-S recombinant) – 1 dose
COVID-19 vaccines listed for emergency use by the WHO:
- US FDA-authorized vaccines above
- AstraZeneca-Oxford (Covishield, Vaxzevria; ChAdOx1-S recombinant) – 2 doses
- Sinopharm (Vero Cell inactivated) – 2 doses
- Sinovac (Vero Cell inactivated) – 2 doses
See the table below for a summary of recommendations for patients who received a COVID-19 vaccination outside of the US:
Resources:
- WHO – Prequalification of Medical Products: Coronavirus Disease
- Regulation and Prequalification: COVID-19 vaccines
- CDC’s Interim Clinical Considerations for Use of COVID-19 Vaccines
- New York Times: COVID World Vaccination Tracker Map
Thank you for keeping Philadelphia safe and healthy! If you have any questions, please email vaccines@phila.gov.
