Recap: RSV Immunization in Outpatient Settings
Recap: RSV Immunization in Outpatient Settings
Thank you to those who attended the RSV townhall for nirsevimab (Beyfortus) immunization in outpatient settings on Tuesday, August 29! In case you missed it, please review important information below:
- PDPH townhall presentation slides
- PDPH townhall meeting recording
- Nirsevimab FAQ
- Nirsevimab planning checklist
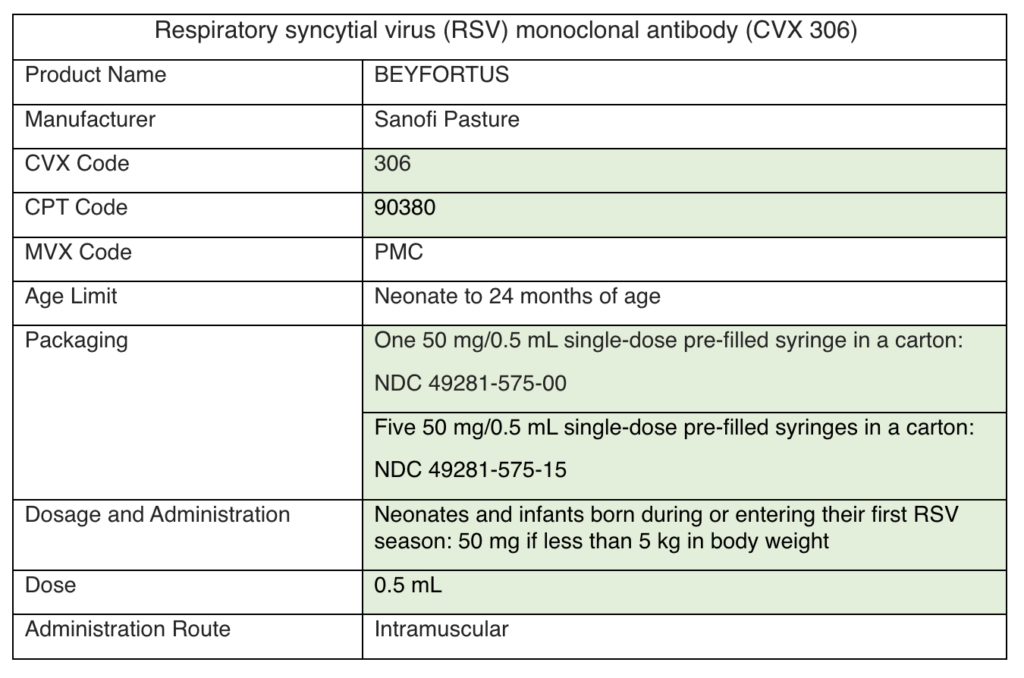
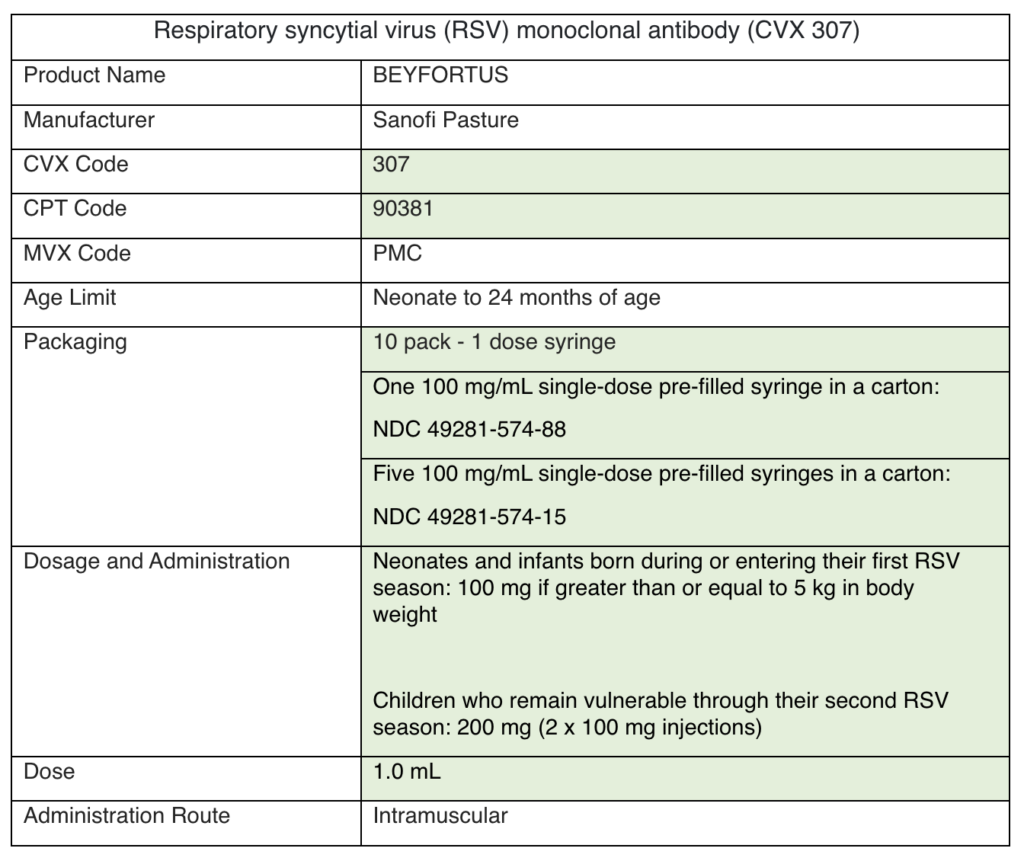
Nirsevimab will be available to order through the Vaccines for Children (VFC) Program this fall. Here are some answers to relevant clinical questions:
Do healthy infants need to be under 8 or 9 months at the time of injection?
Healthy infants need to be under 8 months of age at the time of injection.
What about infants who are at increased risk of severe RSV?
Children aged 8–19 months who are at increased risk of severe RSV disease entering their second RSV season should be immunized.
Any recommendations for co-administration of nirsevimab with COVID-19, flu, and routine vaccines?
Co-administration with other vaccines is acceptable.
Is there a patient information brochure to give to parents?
CDC will have an information sheet similar to a VIS which has not yet been released.
Can nirsevimab (Beyfortus) be administered when a child is ill?
It is up to the doctor’s discretion, and dependant on how ill the infant is.
Does an infant that gets palivizumab (Synagis) needs nirsevimab (Beyfortus)?
An infant that qualifies for palivizumab (Synagis) may need to get a dose if nirsevimab (Beyfortus) is unavailable. Once they receive their nirsevimab, they no longer need to get palivizumab.
View the MMWR and package insert here. Email victor.obeck@phila.gov for more information on ordering nirsevimab through the VFC Program.