Protect Your Patients from Influenza
Protect Your Patients from Influenza
We expect a high number of influenza cases this winter. Vaccination is the most effective tool available to prevent severe respiratory illness and hospitalization. Nationally, there were 200 pediatric flu-related death in the 2023-2024 season, and one pediatric death resulting from flu this season. The ideal time to receive the influenza vaccine is now, before holiday gatherings and influenza peaks. Below, review eligibility recommendations from the CDC and stay updated on flu products available to order through the Vaccines for Children (VFC) program.
Recommendations for Flu Immunization and Co-Administration
CDC recommends:
- Everyone aged six months and older should get an annual influenza vaccination.
- If needed, influenza and other vaccines (e.g., COVID-19, RSV, nirsevimab) may be given at the same visit.
VFC Products Available for the 2024 – 2025 Flu Season
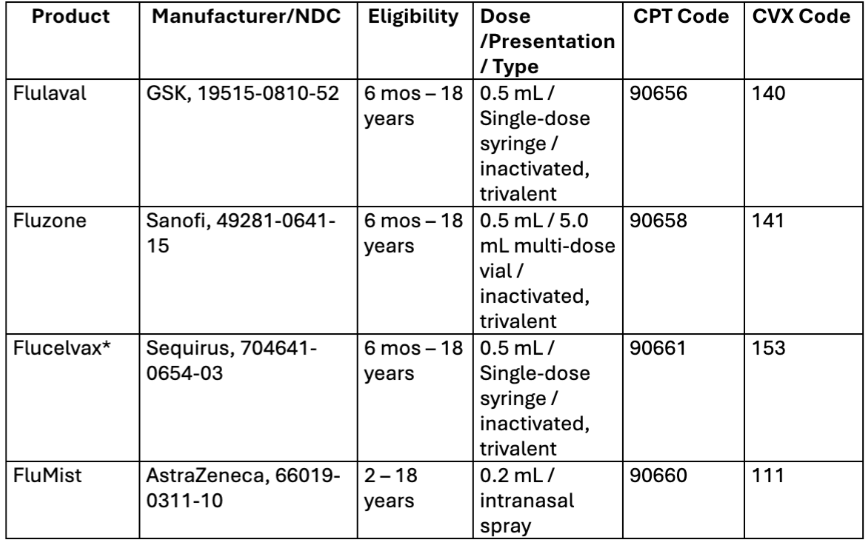
You can order influenza vaccine anytime—no need to wait between orders or complete a reconciliation.
Visit our website for instructions on how to place your order.

