VFC and VFAAR Shipping Delays
VFC and VFAAR Shipping Delays
Our vaccine distribution center is currently working through an unanticipated backlog of VFC and VFAAR orders as a result of having relocated. Due to this please be aware that there are likely to be delays with some vaccine shipments.
For your September and October orders, add 1 to 2 weeks of additional vaccine, if you have space in your storage units. Please add a note to the comments section of the order indicating this intention.
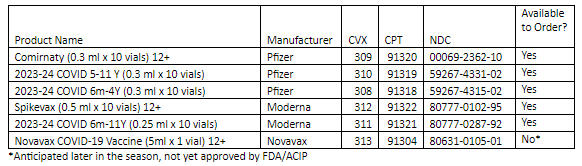
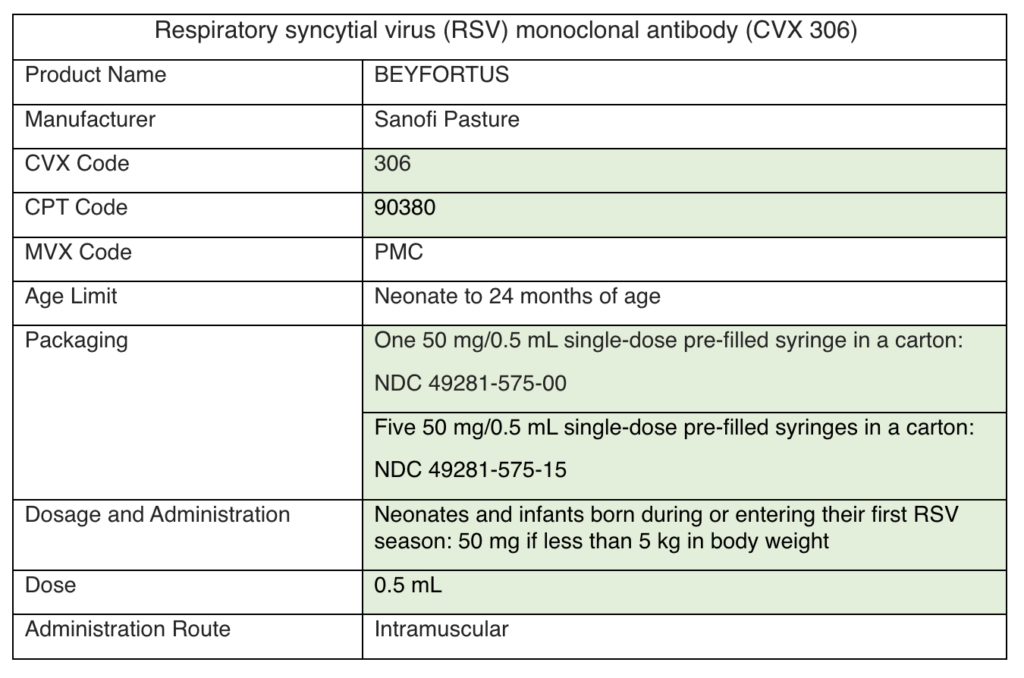
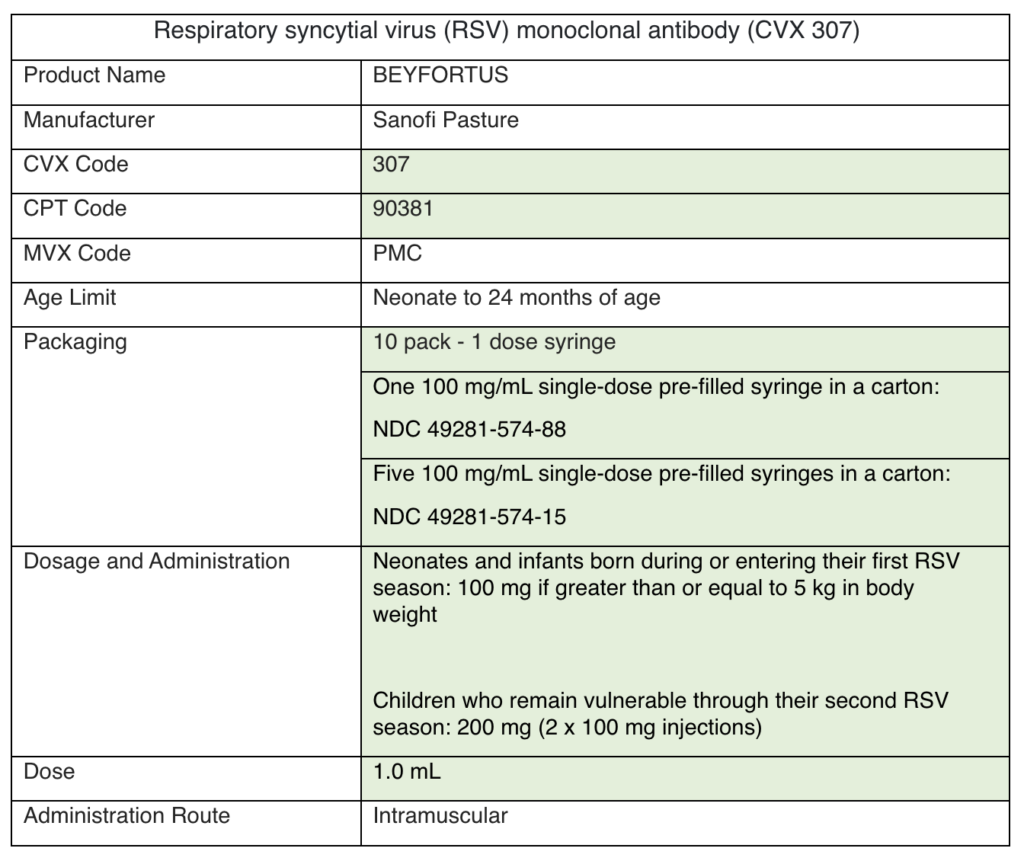
As a reminder, you can order routine vaccines every 25 days (from the last approved order). Use the ordering formula cheat sheet to calculate how much vaccine to order. Continue to order flu vaccine as needed.
We’re here to help! Reach out to us with any questions at DPHProviderHelp@phila.gov.