30 Aug 2023 Integrating RSV Immunization into EHR Systems
Integrating RSV Immunization into EHR Systems
RSV immunization will be available to order through the Vaccines for Children (VFC) program this fall. This newly approved monoclonal antibody, nirsevimab (Beyfortus), will be used to protect newborns and infants born during or entering their first RSV season.
As your facility will likely administer this immunization, we are providing instructions for adding nirsevimab to your EHR system.
Please add nirsevimab to your EHR system. There are two versions of nirsevimab to account for two different doses. See the specifications below. The differences between the two are highlighted in green:
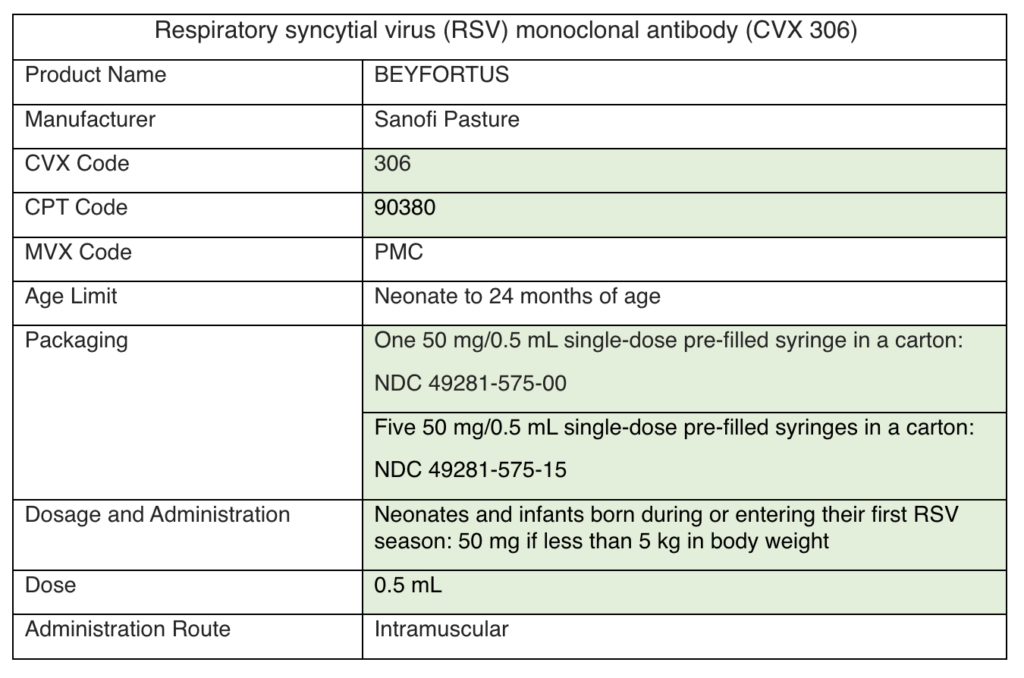
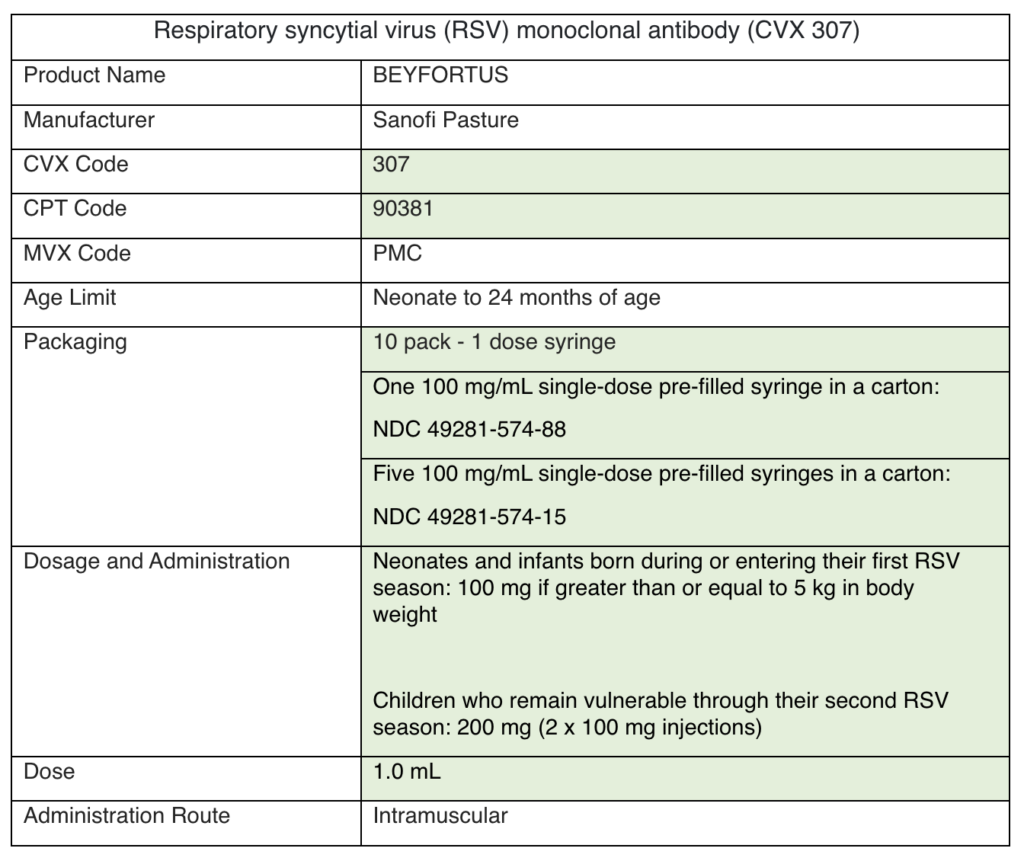
Nirsevimab (Beyfortus) must be reported to PhilaVax in the same way as any other immunization. To accurately report eligibility and funding sources, HL7 messages for nirsevimab administration should adhere to the standard rules for VFC eligibility or ineligibility.
If you are interested, you may work with PhilaVax staff individually to test nirsevimab HL7 messages and ensure complete reporting. To express interest in assistance with this process or for any questions, contact the immunization information system (IIS) interoperability coordinator at beweh.willor@phila.gov.
Please forward this email to any additional contacts who need this information. If you are not the correct person to receive this email, reach out to beweh.willor@phila.gov with the correct contact information.

