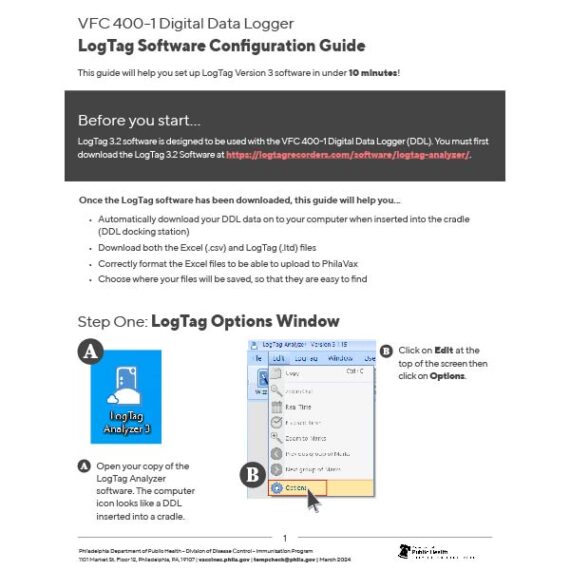
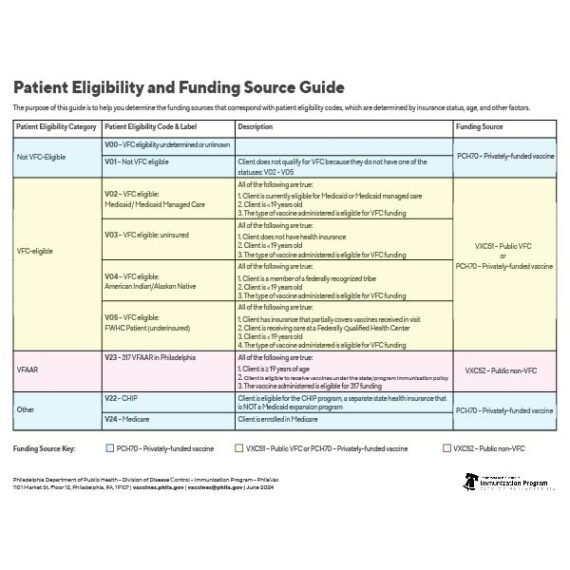
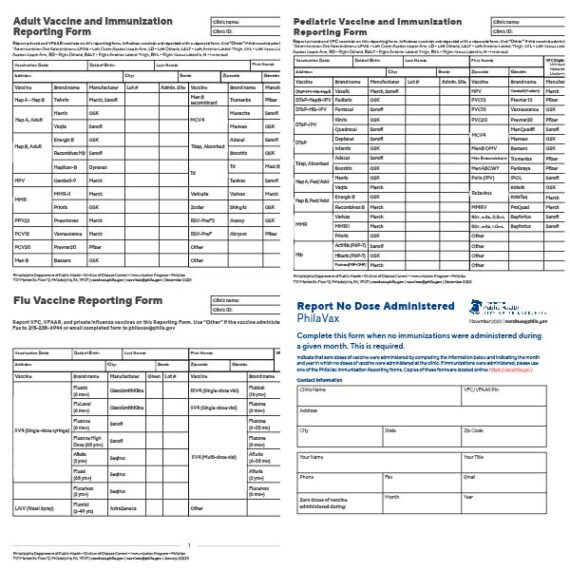
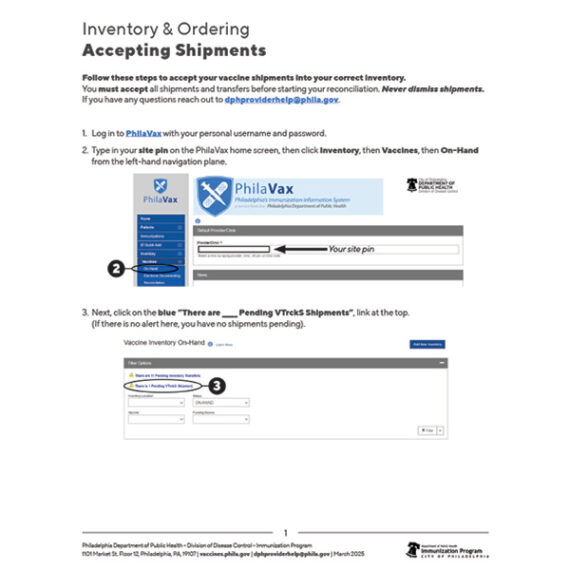
 ';
view
';
view

Quick links
Contact us
Trouble logging in? Forgot password? We can help!
215-685-6784
215-238-6944
Register a clinic
Fill out both forms, then either email or fax them to us at the contact above.