Free Print Materials on the Way!
Free Print Materials on the Way!
We’re sending pediatric and adult materials to your office. Preview some of the print materials that are on the way, and order more in different languages!
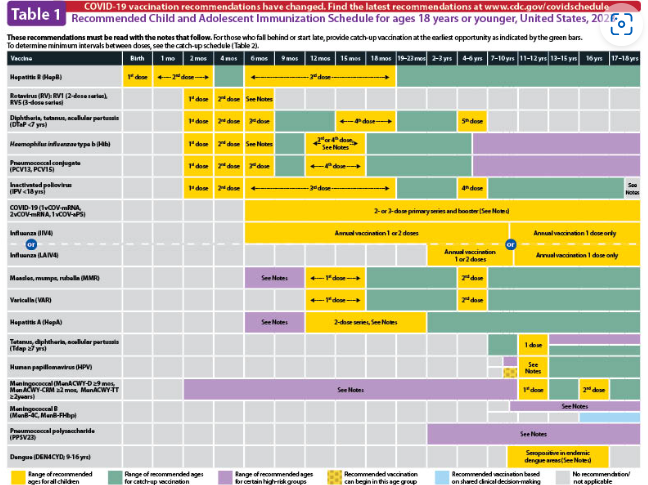
Preview: Laminated Pediatric Vaccine Schedule
Preview: RSV Palm Card for Adults

Visit our website to order more free print materials for your office.
Available Print Materials
Ordering Tip
Once you have found the materials you want, make a note of their ID#s and head over to the corresponding online ordering form. We recommend opening the online ordering form in a separate tab. This will let you easily switch between the online ordering form and the image galleries.
If you have questions or need help with the ordering process, email us at vaccines@phila.gov.