2023-2024 Pfizer (5 yrs-11 yrs and 12+) COVID-19 Vaccines No Longer Available
2023-2024 Pfizer (5 yrs-11 yrs and 12+) COVID-19 Vaccines No Longer Available
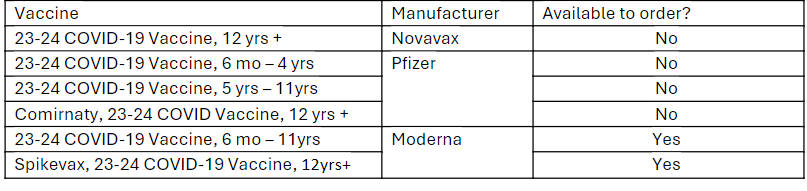
As of Monday, June 10, 2024, the Pfizer 2023-2024 COVID-19 vaccine, 5 yrs – 11 yrs, and Comirnaty (Pfizer), 2023-2024 COVID-19 vaccine, 12 yrs + products are no longer available to order through the Vaccines for Children (VFC) and Bridge Access Programs.
Continue to order and administer 2023-2024 COVID-19 vaccines until they reach their beyond use date (BUD), expire, or new product becomes available. We’ve seen summer peaks in past years, so individuals aged 6 months and above who have not received a dose should continue to be vaccinated.
See below for details on the availability of the different products. If your preferred product is no longer available, change to an alternate product and continue to offer and administer COVID-19 vaccine to patients.
Dosing Schedule: 2023-2024 COVID-19 Vaccine

For more details, review the CDC’s Interim 2023-2024 COVID-19 Immunization Schedule for Persons 6 Months of Age and Older.
Available Products (updated 6/10/2024): 2023-2024 COVID-19 Vaccine
Upcoming Changes in COVID-19 Vaccine Product Availability
CDC anticipates upcoming changes in the availability of COVID-19 vaccine products.
Here’s what we know now about the upcoming changes in 2023-2024 COVID-19 product availability:
- The latest expiration date for the Pfizer (6 mo – 4 yrs) product is 7/31/2024.
- Moderna (6 mo – 11 yrs and 12+) product has expiration dates through late September.
Be sure to check expiration dates before administering any vaccine.
Other Considerations for COVID-19 Vaccine Product Expiration
- The expiration dates indicated above are the latest 2023-2024 expiry. Your office may have vaccine products that expire sooner.
- The beyond use date (BUD) supersedes the manufacturer’s expiration date.
We will send additional messages to remind you about these expiration dates as they occur, provide guidance for inventory management, and inform you of other product updates as we are notified.
If you have questions about COVID-19 vaccine availability, please reach out.
For VFC, email Victor Obeck at victor.obeck@phila.gov.
For the Bridge Access Program, email Nyandra McFadden at nyandra.mcfadden@phila.gov.