Beyfortus (Nirsevimab) Availability & Inventory Management
VFC ordering for the 2024 – 2025 Beyfortus (nirsevimab) season will begin this week.
The CDC is releasing VFC Beyfortus (nirsevimab) in waves throughout the 2024-2025 season. Since a limited number of doses will be available in each wave, the Immunization Program will work with sites throughout the season to place orders for Beyfortus as doses are made available. Your site will be contacted via email with a specific timeframe and amount that you are permitted to order. Do not attempt to order outside of your timeframe. Orders placed outside your timeframe will be rejected. Orders may also be adjusted to ensure adequate and equitable distribution of the available doses throughout the city.
Make sure that your contact information is up to date and that you are checking your email regularly as turnaround times are short.
Please contact our VFC Coordinator (victor.obeck@phila.gov) or our Ordering Team (DPHproviderhelp@phila.gov) with any questions.
Beyfortus (Nirsevimab) Guidance
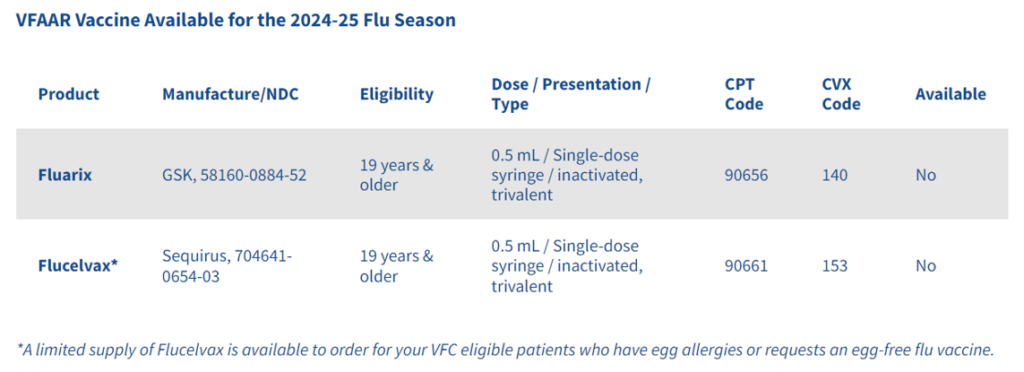
Beyfortus (nirsevimab) can be administered beginning October 1, 2024. If you have doses remaining from the previous season, they are still viable.
Dosage and Administration
RSV comes in two dosages:
Both dosages are administered as an intramuscular injection with a single-dose, pre-filled syringe, and should be ordered to properly administer by weight.
Eligible Patients
- Infants aged <8 months born during or entering their first RSV season
- 1 dose of nirsevimab (50 mg for infants <5 kg and 100 mg for infants ≥5kg)
- Children aged 8-19 months who are at increased risk of severe RSV disease and entering their second RSV season
- 200 mg nirsevimab, administered by two 100 mg syringes
Click here to view more information about which children are at increased risk and should be vaccinated during their second season
Click here to view a flowchart to aid providers in determining the appropriate dose of Beyfortus (nirsevimab) for patients.
Storage and Handling
Store refrigerated between 36°F to 46°F (2°C to 8°C) through the expiration date. Nirsevimab may be kept at room temperature 68°F to 77°F (20°C to 25°C) for a maximum of 8 hours. After removal from the refrigerator, nirsevimab must be used within 8 hours or discarded.
Reporting Adverse Events
Nirsevimab is the first drug product to be included in the VFC program. If nirsevimab is administered: