Digital Data Logger Download Reminder During the Holiday Season
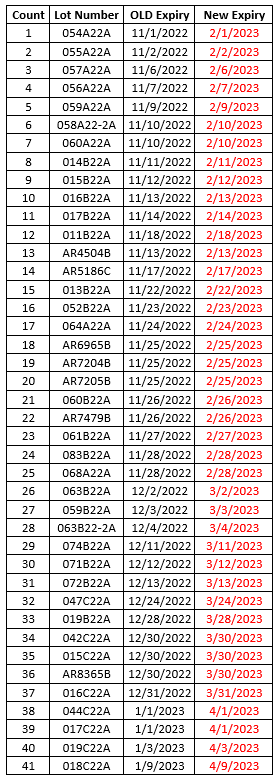
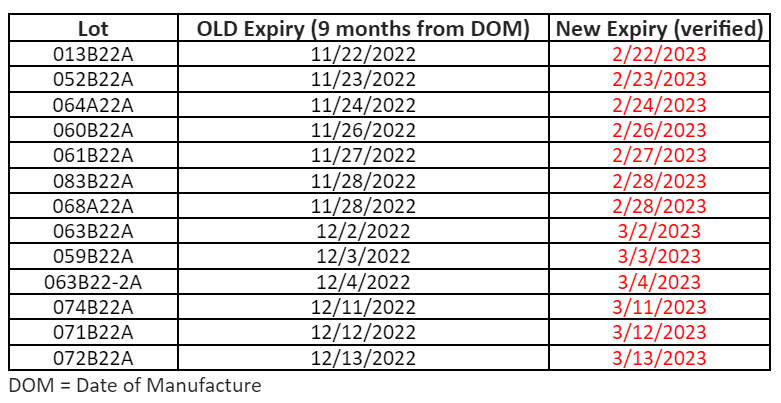
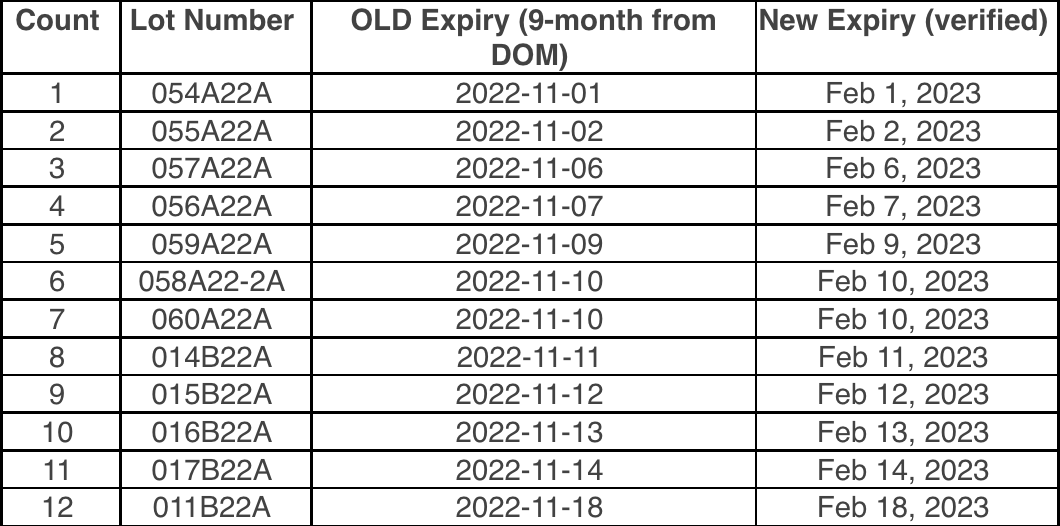
To make sure that your vaccines stay properly monitored over the busy holiday season, please be aware of the date indicator on your digital data loggers (DDLs). To prevent any gaps in temperature monitoring, please download your DDL files if the date indicator is approaching the 30 day capacity. With many offices having adjusted holiday hours or staff members taking time off, the 30 day capacity on your DDL could be reached.
To prevent this, we suggest that you download your DDL files before the holidays, especially if your office will be closed. This may mean downloading your DDL earlier than you normally would. This will ensure that they continue to record temperature readings for your storage units into the New Year.
After you download your DDL files, remember to:
1) Email both your LTD (yellow and blue) and CSV (green) files to TempCheck@phila.gov
2) Upload your CSV files into the Clinic Tools module in PhilaVax
If you need assistance or have additional questions, please contact our Storage and Handling department at TempCheck@phila.gov.