Vaccine Temperature Monitoring: New Paper Temperature Logs Available
Vaccine Temperature Monitoring: New Paper Temperature Logs Available
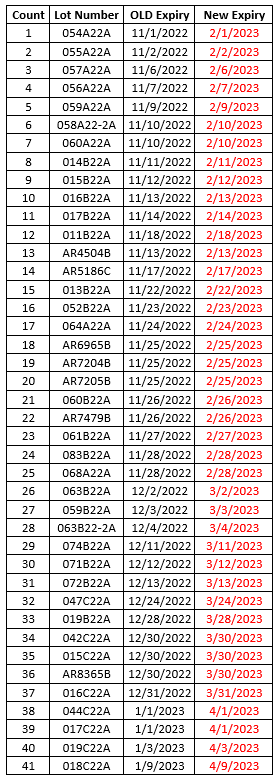
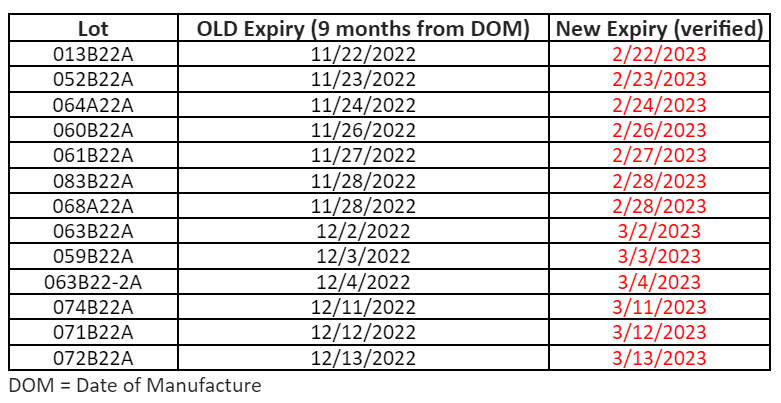
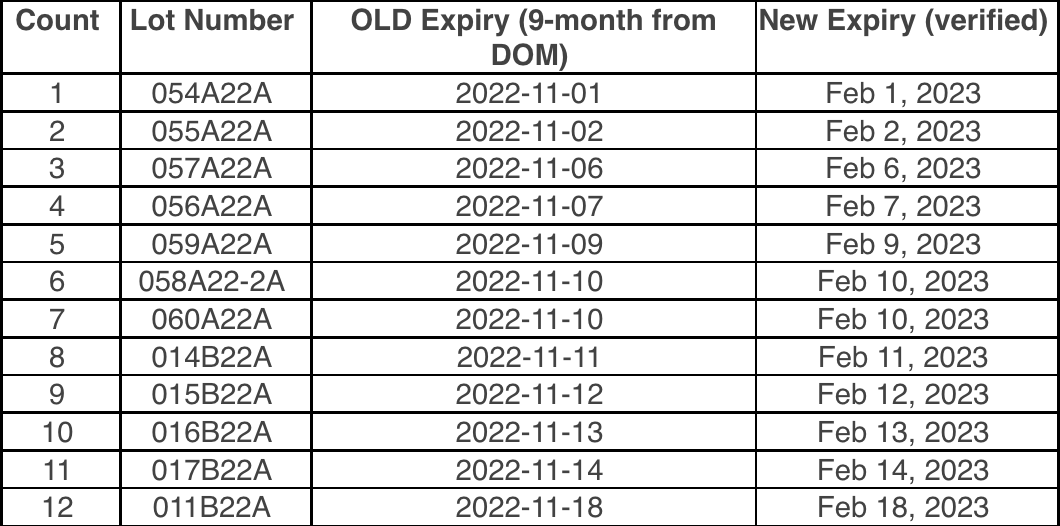
To ensure that the vaccine at your site is viable when administered to patients, consistent temperature monitoring is an important part of participating in the program. Our program requires sites to maintain active and passive monitoring. The passive monitoring is recorded by the Digital Date Logger (DDL) and reported to our program regularly. The active monitoring is recorded on the paper temperature logs.
Please download and review the updated documents below:
Fridge Temperature Log (Celsius)
Fridge Temperature Log (Fahrenheit)
Freezer Temperature Log (Celsius)
Freezer Temperature Log (Fahrenheit)
Please use the new logs and review the instructions to make sure you are documenting temperatures correctly.
Paper temperature logs are a requirement for the VFC, VFAAR, and COVID-19 programs and must be kept on file for at least 3 years.