Nirsevimab (Beyfortus) and COVID-19 Updates
Nirsevimab (Beyfortus) and COVID-19 Updates
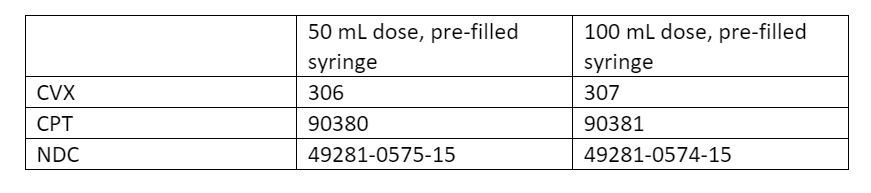
Nirsevimab
Due to high demand and limited supply cited by the CDC, nirsevimab (Beyfortus) ordering through VFC has been put on hold as of October 17, 2023. The CDC is working with Sanofi to secure a supply of this immunization within the next 2 – 3 weeks.
We will provide an update when nirsevimab becomes available for ordering. Please continue to immunize eligible children with your current supply.
COVID-19
For children under 5, access to COVID-19 vaccine is currently limited as most pharmacies are not vaccinating this age group. As a provider enrolled in the Vaccines For Children (VFC) program, here’s how to ensure these kids get the vaccine they need:
- Order COVID-19 vaccine through the VFC program
- Borrow VFC doses of COVID-19 vaccine to immunize your privately and CHIP insured patients while you wait for COVID-19 supply from the manufacturers*
*To request a borrowing form and learn more about the guidelines of this limited-time option, contact our VFC coordinator at victor.obeck@phila.gov. We highly encourage continuing to offer COVID-19 vaccine at every opportunity.
What does commercialization mean for VFC providers?
The Department of Public Health’s federal COVID-19 program has formally ended. COVID-19 vaccines are now part of the routine immunization schedule.
With the exception of certain specialty providers, all Vaccines for Children (VFC) providers are required to stock COVID-19 vaccine inventory for both privately/CHIP insured patients and VFC eligible patients.
Learn more in this blog post that consolidates recent COVID-19 vaccine updates and lists available COVID-19 products.
Resources
Nirsevimab:
Recommendations and ordering guidance
MMWR
FDA page
Package insert
Immunization Information Statement (use this instead of VIS!)
Product and ordering information (AAP)
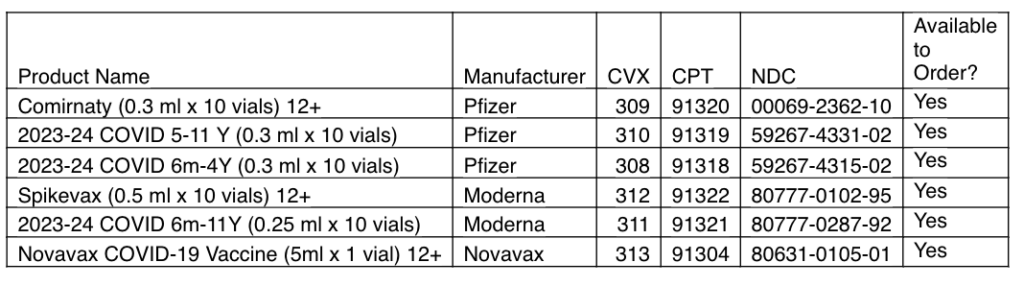
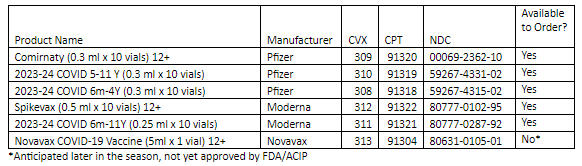
COVID-19:
Pfizer 2023-24 COVID-19 Vaccine
Comirnaty 12+
2023-24 COVID Vaccine 5 yrs – 11 yrs
2023-24 COVID Vaccine 6 mo – 4 yrs
Moderna 2023-24 COVID-19 Vaccine
Spikevax 12+
2023-24 COVID Vaccine 6 mo – 11 yrs
Have other questions about nirsevimab, COVID-19, or VFC? Contact our VFC coordinator at victor.obeck@phila.gov.