Additional VFAAR Flu Vaccine Now Available for Ordering
Additional VFAAR Flu Vaccine Now Available for Ordering
- VFAAR
- Flulaval prefilled syringes
- Flucelvax prefilled syringes
Monkeypox Vaccine Deliveries Paused for Thanksgiving
Monkeypox vaccine deliveries will be paused during the week of Thanksgiving, November 21 – 25, 2022. The last delivery for monkeypox vaccine will be on Monday, November 21.
Ordering will remain open this week until Thursday, by 5pm. Remember to submit your temperatures to tempcheck@phila.gov and complete a reconciliation of vaccine on hand for your order to be approved.
Click the button below to submit your order request for monkeypox vaccine.
Deliveries for monkeypox vaccine will resume on the week of November 28.
Thank you for keeping Philadelphia safe and healthy! If you have any questions, please contact Kenya Mack at Kenya.Mack@phila.gov.
On Monday, November 14 through Tuesday, November 15, PDPH will open pre-ordering in Philavax for a limited amount of the updated (bivalent) Pfizer-BioNTech COVID-19 vaccine for people 12 years of age or older in single-dose vials. Deliveries will begin several days later. Single dose vials will not be available for ordering through Monday.com – partial ordering – or pickup.This limited rollout is intended to expand the number of locations that offer the updated vaccine. Only sites that would otherwise not be able to utilize the standard six-dose per vial bivalent Pfizer will be approved to order single dose vials. Large health systems and sites with regular patient volumes/throughput should continue to order the six-dose per vial bivalent Pfizer.Ancillary kits will not be distributed with orders of single-dose vials. Single-dose vials do not require the use of low-dead-volume (LDV) syringes.Quick Facts:Single-Dose Vial Pfizer Bivalent Booster (Ages 12+)• NDC: 59267-1404-02• Configuration: 10 single-dose vials/carton• Minimum quantity per order: 50 doses• Maximum quantity per order: 150 doses• Product dimensions: 1.457 in length × 1.535 in width × 3.504 in height• Storage and handling: Same as other Pfizer tris products (e.g., store at ultra-low temperature until expiry, may refrigerate up to 10 weeks within expiry period)• No ancillary kits included with single-dose vial orders
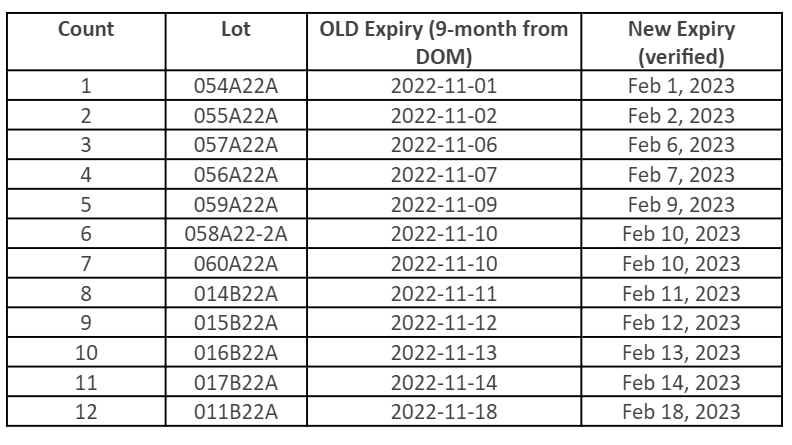
FDA has approved a shelf-life extension for the following Moderna10 products, which have been extended by three months. Below, find a table with updated expiration dates for the specific lots impacted by the extension. For the most current information, please visit the Moderna website.
The Philadelphia Department of Public Health’s COVID-19 Matchmaking Program would like to send a huge thank you for your time, commitment, and expertise you’ve given in partnering with vaccination clinics to provide vaccine. After careful consideration, we have determined to end the program effective November 4, 2022. 57 vaccination clinics were successfully matched for the duration of PDPH’s COVID-19 Matchmaking Program, and 77 providers were enrolled. These vaccinations clinics included community-based clinics, health fairs, and cultural events to name a few!If patients are looking to get vaccinated at their earliest convenience, you may direct them to use this link to find a facility in close proximity to them. Again, we thank you for your contributions and efforts to partner with vaccinations clinics to serve vaccine. You have made an immense difference through your dedication and continued support!
The Philadelphia Health Department is looking to identify provider offices and clinics that administer monkeypox JYNNEOS vaccine to non-patients and offer walk-in hours. If you are a monkeypox vaccine provider, please fill out the brief survey.
Health Department Publishes Request for Proposal for HIV Testing in Pharmacies
PHILADELPHIA—The Health Department is making four to six grants of up to $75,000 available to increase the availability of low-barrier HIV testing and connection to care services in select priority zip codes in Philadelphia. The request for proposals is part of the Health Department’s community-driven Plan to End the HIV Epidemic in Philadelphia, which envisions decreasing new HIV infections by 75% by 2025 and by 90% by 2030. Grant submissions may be made by applicants who operate pharmacies in the listed priority zip codes, and may address HIV testing, information distribution, linkage to care, and participation in training.
The accessibility of pharmacies for HIV testing presents a unique opportunity for pharmacists to contribute to the identification of undiagnosed HIV. An estimated 90% of urban consumers live within two miles of a pharmacy. A CDC-funded feasibility study offering rapid, point-of-care testing in community pharmacies and retail clinics found that pharmacies and retail clinics represent a vast, largely untapped potential for the delivery of HIV testing in settings that are more accessible and, for some people, less stigmatizing than traditional testing.
With this RFP, the Health Department’s goal is to expand opportunities for HIV testing in neighborhoods in Philadelphia with the highest incidence of persons newly diagnosed with HIV in 2019 through community-based retail pharmacies.
Eligible applicants are independent and non-independent pharmacies currently operating in the priority areas listed in this request for proposals or with appropriate justification and prior approval of the Department in an immediately adjacent ZIP code neighborhood. Based on local analysis of recent data on HIV testing, newly diagnosed HIV, and HIV testing resources by zip code, the Health Department has identified 13 priority zip codes for this RFP.
To learn more about the request for proposals, all documentation is available on the eContractPhilly website, under Opportunity 21221013101712. The Health Department’s Community Plan to End the HIV Epidemic in Philadelphia can be downloaded here.