29 Sep 2023 COVID-19 Vaccine Commercialization
COVID-19 Vaccine Commercialization
Tuesday, September 12, 2023, marked the beginning of COVID-19 commercialization. Here are some important changes to be aware of in this new period of COVID-19 vaccination:
- The Department of Public Health’s COVID-19 program has formally ended.
- Bivalent COVID-19 vaccines are no longer authorized for use.
- The Advisory Committee on Immunization Practices (ACIP) approved the use of updated (monovalent) COVID-19 vaccines.
- Providers can order the updated COVID-19 vaccine for privately insured patients by directly contacting the manufacturers for each product (see below for more information).
- With the exception of certain specialty providers, all Vaccines for Children (VFC) providers are required to stock COVID-19 vaccine inventory for both privately/CHIP insured patients and VFC eligible patients.
- Philadelphia vaccine providers are encouraged to enroll in the Bridge Access Program to provide updated COVID-19 vaccines for uninsured or underinsured adults 18 years and older.
The new COVID-19 vaccine products are monovalent (single), Omicron variant XBB.1.5. Under the new recommendation, everyone over 6 months is eligible for an updated COVID-19 vaccine this fall.
Providers can place COVID-19 vaccine orders for VFC eligible patients and/or the Bridge Access Program eligible patients through the PhilaVax immunization information systems (IIS). A step-by-step ordering guide is available to assist with this.
Recommendations for COVID-19 Vaccine
- Everyone ages 5 years and older is recommended to receive 1 dose of a 2023-24 mRNA COVID-19 vaccine.
- Children ages 6 months – 4 years should complete a multi-dose initial series (2 doses of Modera or 3 doses of Pfizer mRNA COVID-19 vaccine) with at least one dose of the 2023-24 COVID-19 vaccine.
- People who are moderately or severely immunocompromised should complete a 3 dose initial series with at least one dose of the 2023-24 COVID-19 vaccine and may receive 1 or more additional 2023-24 COVID-19 vaccine doses.
- Bivalent mRNA COVID-19 vaccines are no longer recommended.
Available Products
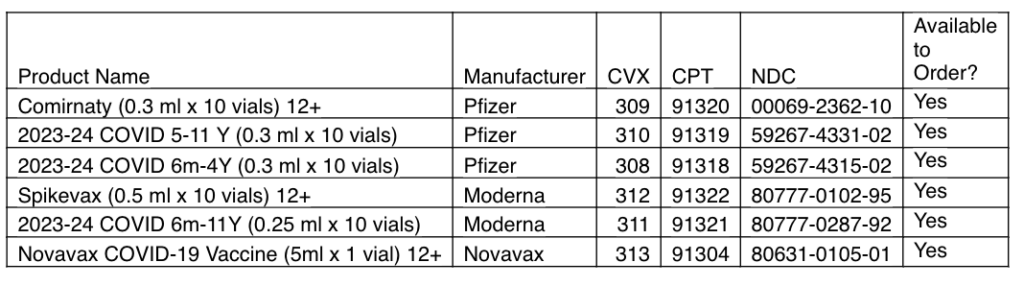
Pfizer 2023-24 COVID-19 Vaccine
Comirnaty 12+
2023-24 COVID Vaccine 5 yrs – 11 yrs
2023-24 COVID Vaccine 6 mo – 4 yrs
Moderna 2023-24 COVID-19 Vaccine
Spikevax 12+
2023-24 COVID Vaccine 6 mo – 11 yrs
Additional Resources
If your office needs assistance placing an order or has questions, please reach out to dphproviderhelp@phila.gov.
Purchase Vaccine for Privately Insured Patients
To procure vaccine on the private market, please contact your local sales representative:
- Pfizer: Brian Bengston, Brian.Bengston@pfizer.com
- Moderna: Lynne Timby, Lynne.Timby@modernatx.com
- Novavax: Rich Aceto, RAceto@novavax.com
Guidance for Patients Regarding Vaccine Availability
Patients are having some initial difficulty finding updated COVID-19 vaccine, especially pediatric doses. This is due to delays in shipping from manufacturers. The Health Department published recommendations for Philadelphians seeking updated COVID-19 vaccine doses. Read the recommendations here and consider sharing them with your patients.


