14 Nov 2025 Updated Clinical Guidance for COVID-19 Vaccine
The CDC released updated clinical guidance for the 2025-2026 COVID-19 vaccine.
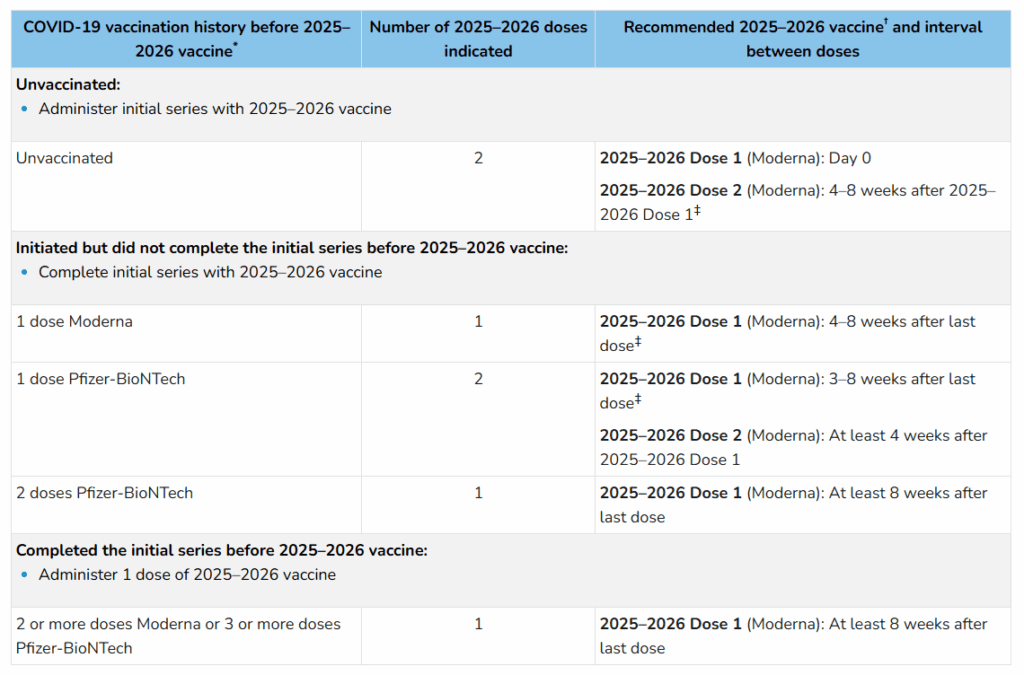
- Previously, all unvaccinated children younger than 5 years were recommended to receive 2 doses of 2025-2026 Moderna vaccine as their primary series.
- Now, children aged 6-23 months who are unvaccinated are recommended to receive 2 doses as their primary series. In addition, children aged 6-23 months who previously received 1 dose of Pfizer vaccine should also receive 2 doses of 2025-2026 Moderna vaccine. As a reminder, Pfizer vaccine is no longer available for children aged 6 months-4 years.
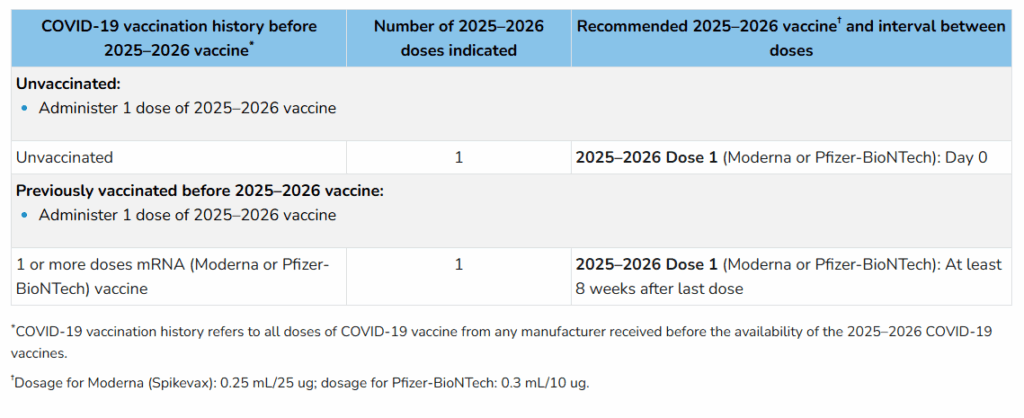
- It is also recommended that children aged 2 years (24 months) and older receive only 1 dose of the 2025-2026 Moderna vaccine, even if previously unvaccinated.
- If given 2025-2026 Novavax vaccine, children aged 12+ are recommended to receive 1 dose, even if previously unvaccinated.
Review the tables (previewed below) on this CDC webpage for a deeper dive into these changes.



For any questions about this updated guidance, please contact us at vaccines@phila.gov.


