09 Nov 2022 Moderna Shelf Life Extension
Posted at 13:44h
in
COVID-19
Moderna Shelf Life Extension
FDA has approved a shelf-life extension for the following Moderna10 products, which have been extended by three months. Below, find a table with updated expiration dates for the specific lots impacted by the extension. For the most current information, please visit the Moderna website.
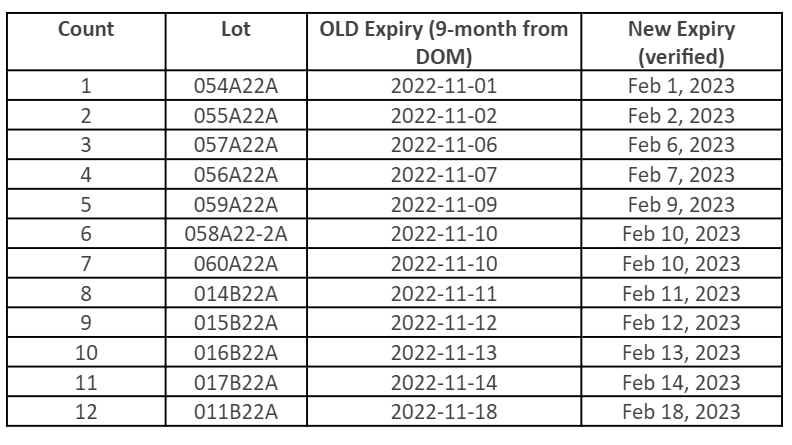
With the
Moderna Vial Expiration Checker, Moderna also allows vaccine providers to look up expiration dates by lot number, which includes the new expiration dates listed above. To find the expiration date, locate the lot number printed on the carton and/or vial and enter it into the lot number search field on the website, then press “submit.”



