Temperature Reporting for Online Vaccine Ordering
Temperature Reporting for Online Vaccine Ordering
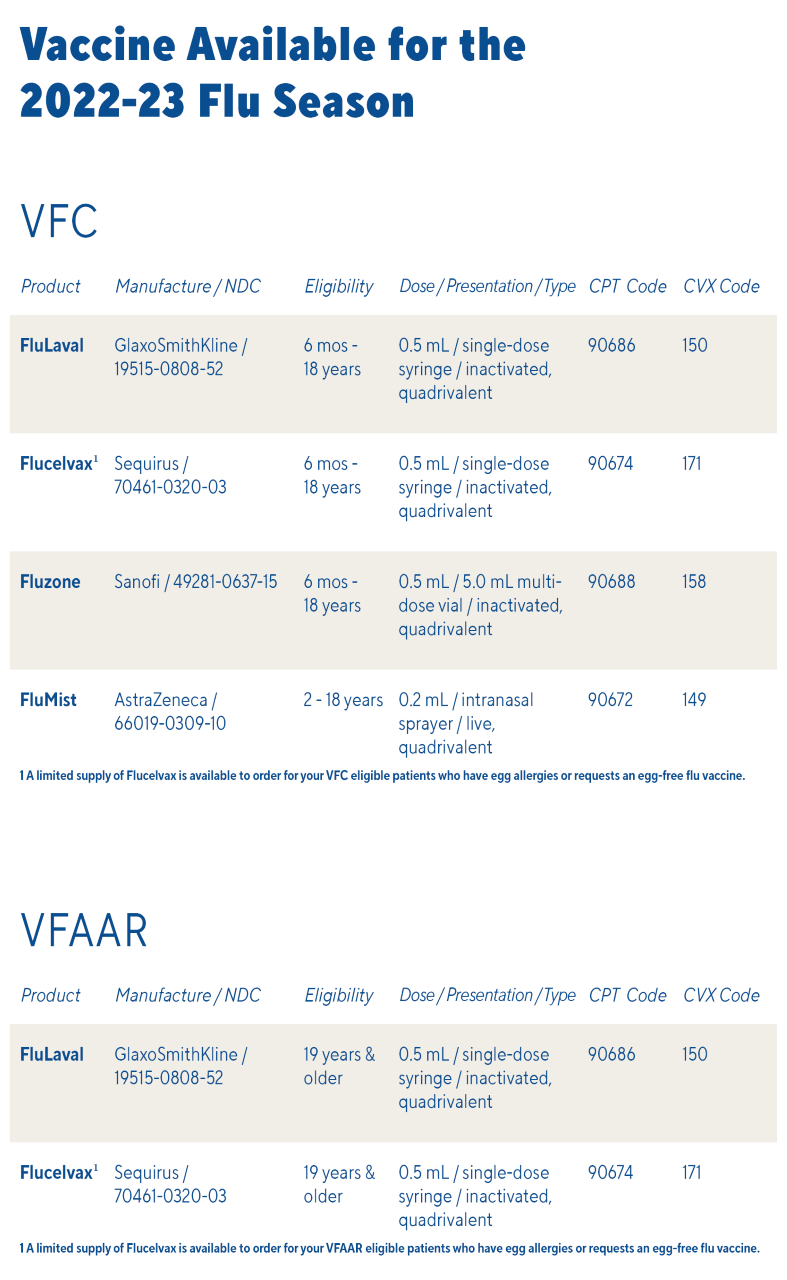
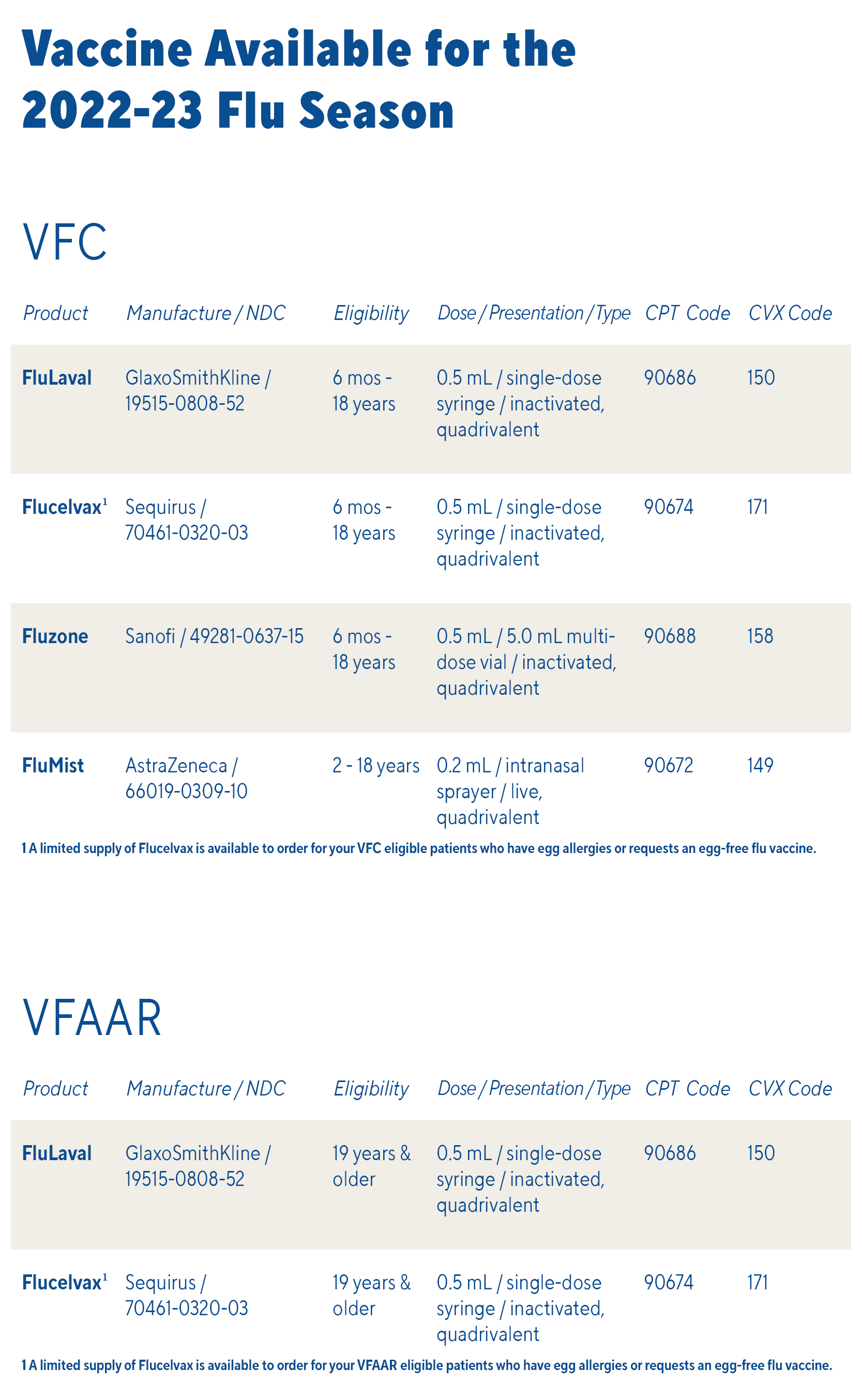
Temperature logs must be submitted on the same day as any order placed for federal vaccine (VFC, VFAAR, flu, COVID-19, or monkeypox)!
Temperature logs are to be uploaded in PhilaVax and emailed to tempcheck@phila.gov. The logs must show that the unit is within the appropriate temperature ranges and that there have been no alarms. This reporting guide can assist you in submitting your DDL temperature files.
For more information on ordering and how to upload your temperature log, please attend the next online ordering training on November 10, 2022 from 10:00AM – 11:00AM EST. Click the link below to register for the training.
If your site uses a privately purchased DDL or cannot upload in PhilaVax, please contact tempcheck@phila.gov.
Thank you for keeping Philadelphia safe and healthy!