14 Sep 2023 COVID-19 Vaccine Available to Order Through VFC and the Bridge Access Program
COVID-19 Vaccine Available to Order Through VFC and the Bridge Access Program
On September 12, 2023, ACIP approved the use of updated COVID-19 vaccines. The new products are monovalent (single), Omicron variant XBB.1.5. Under the new recommendation, everyone over 6 months is eligible for an updated COVID-19 vaccine this fall.
This marks the start of commercialization. VFC sites are now required to stock COVID-19 vaccine, for both your privately/CHIP insured patients and your VFC eligible patients.
Recommendations
- Everyone ages 5 years and older is recommended to receive 1 dose of a 2023-24 mRNA COVID-19 vaccine.
- Children ages 6 months – 4 years should complete a multi-dose initial series (2 doses of Modera or 3 doses of Pfizer mRNA COVID-19 vaccine) with at least one dose of the 2023-24 COVID-19 vaccine.
- People who are moderately or severely immunocompromised should complete a 3 dose initial series with at least one dose of the 2023-24 COVID-19 vaccine and may receive 1 or more additional 2023-24 COVID-19 vaccine doses.
- Bivalent mRNA COVID-19 vaccines are no longer recommended.
Starting, today, Thursday September 14, sites are able to order COVID-19 vaccine for VFC and/or the Bridge Access Program through PhilaVax. View the step-by-step ordering guide.
Available Products
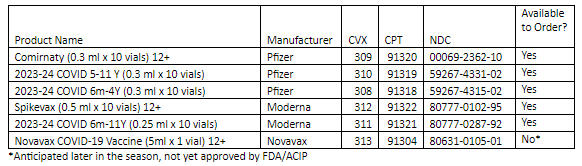
- Pfizer 2023-24 COVID-19 Vaccine
- Comirnaty 12+
- 2023-24 COVID Vaccine 5 yrs – 11 yrs
- 2023-24 COVID Vaccine 6 mo – 4 yrs
- Moderna 2023-24 COVID-19 Vaccine
- Spikevax 12+
- 2023-24 COVID Vaccine 6 mo – 11 yrs
Additional resources
If your site needs assistance placing an order or has questions, please reach out to dphproviderhelp@phila.gov.
To procure vaccine on the private market, please contact your local sales representative:
- Pfizer: Barry Brandt, Barry.Brandt@pfizer.com
- Moderna: Lynne Timby, Lynne.Timby@modernatx.com
- Novavax: Rich Aceto, RAceto@novavax.com
Thank you for keeping Philadelphia safe and healthy. If you have any general questions, please email vaccines@phila.gov.


