20 Dec 2023 New Refrigerated Pfizer 12+ COVID-19 Vaccine
New Refrigerated Pfizer 12+ COVID-19 Vaccine
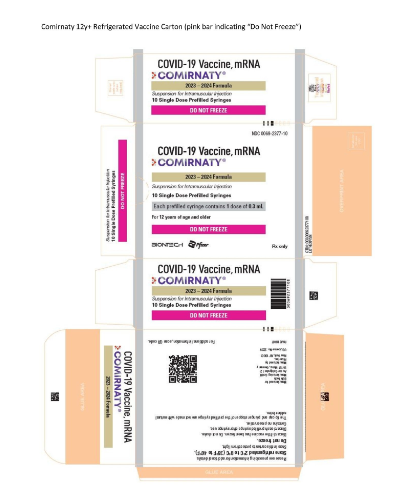
A new presentation of Comirnity (Pfizer COVID-19 vaccine for 12+ year olds) (NDC 00069-2377-10) is now available to order.
The new presentation:
- is a carton of 10 prefilled syringes.
- is a refrigerated formulation that must be stored between 2°C and 8°C (36°F and 46°F). Do not store at ultra-cold or standard freezer temperatures.
- can be used through the expiration date printed on the carton and syringe labels.
The old presentation of Comirnity (Pfizer COVID-19 vaccine for 12+ year olds) (NDC 00069-2362-10) is no longer available to order through the VFC and Bridge programs.
Before ordering or administering the new product, ensure that Comirnity (Pfizer COVID-19 vaccine for 12+ year olds) (NDC 00069-2377-10) has been added to your electronic medical record (EMR) so that administered doses are reported to the PhilaVax IIS.
See below for a picture of the new vaccine presentation. The carton includes a bright pink stripe with the words “DO NOT FREEZE.”
Continue to use any doses of the old Pfizer 12+ vaccine (NDC 00069-2362-10) on hand until it is consumed, expired, or has been stored at 2°C to 8°C for longer than the allowable 10 weeks.
Your site may have both presentations on hand for a period of time. If this occurs, please ensure that doses are clearly labeled and stored appropriately to prevent potential errors.


