07 Dec 2022 Moderna Shelf-Life Extensions
Moderna Shelf-Life Extensions
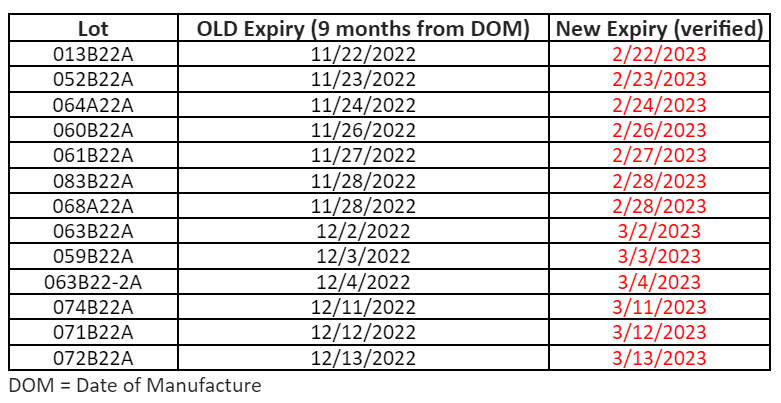
FDA has approved a shelf-life extension for the following Moderna monovalent products. Below, find a table with updated expiration dates for the specific lots impacted by the extension.
With the Moderna Vial Expiration Checker, Moderna also allows vaccine providers to look up expiration dates by lot number, which includes the new expiration dates listed above. To find the expiration date, locate the lot number printed on the carton and/or vial and enter it into the lot number search field on the website, then press “submit.”
When checking existing vaccine inventory, be sure to apply these new expiration dates.
For additional information, view the CDC overview of Moderna vaccine.


