07 Aug 2023 Moderna COVID-19 Vaccine Shelf-Life Extension
Moderna COVID-19 Vaccine Shelf-Life Extension
The FDA has approved Moderna’s request for a 3-month shelf-life extension (SLE) of all Moderna COVID-19 vaccines for under 6 years old. The relevant lot numbers are listed in the table below.
Moderna has updated their web-based Vial Expiration Checker tool to reflect the extension of the four lot numbers below. Only the lot numbers below are included in this SLE approval.
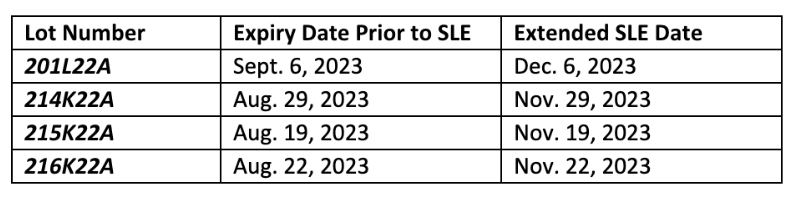
We encourage you to record these updates for management of your inventory and continue weekly monitoring of all COVID-19 vaccine expiration dates using the manufacturers’ online expiry checking tools:
Moderna Vial Expiration Checker


