Advisories
2020 Enrollment begins February 3rd
2020 Enrollment begins February 3rd
Happy new year! The Philadelphia Immunization Program annual Vaccines for Children (VFC) and Vaccines for Adults at Risk (VFAAR) enrollment opens on Monday, February 3rd.Start the new year off on the right foot. Complete your VFC/VFAAR enrollment on time.
This yearâs enrollment period runs from February 3rd to February 28th. During this time all VFC/VFAAR sites must log in and complete the online enrollment. Annual enrollment is required to receive federally funded vaccines regardless of if it’s your first year or hundredth! Don’t wait until the end of February. Login and get started on Monday, Februar2019-2020 Flu Paper Reporting Forms
Update your Digital Data Logger software today
Update your Digital Data Logger (DDL) software today
Please update to the latest version of the LogTag software, which makes small but important changes to how the digital data loggers (DDL) work and prevent future compatibility issues.
This update will not affect downloading processes or your daily responsibilities and will take about 1 minute to do. Please do this update by Friday, 1/25.
How to update your software
To update your software:
- Visit https://logtagrecorders.com/software/lta2/download/
- Download Installer: Itanalyzer_2.9r8.exe
- Run the program you downloaded
- Complete this electronic form, https://goo.gl/forms/oGG29vvXweWvenxx2, to let us know when you have successfully downloaded the software or to let us know about issues you experienced.
If you need administrative or IT permission to download software, please contact your IT department as soon as possible.
If you have any questions about this update please contact:
Alexis Bridges and Adam Howsare
Storage & Handling Coordinators
Phone: 215-685-6777
VFC and VFAAR Re-Enrollment is Coming
VFC/VFAAR Re-enrollment is right around the corner!
The Philadelphia VFC/VFAAR annual re-enrollment period is November 1-30, 2018. Re-enrollment is a requirement for the VFC/VFAAR programs. Failure to complete the re-enrollment form will result in un-enrollment from the program.
Weâre very excited to announce that for the first time, enrollment will be done electronically through PhilaVax. This will save you time and allow us to process enrollment forms quicker.
To get you ready for re-enrollment, complete these three steps now (if you have done so already).
- Complete the Clinic Tools Training
The electronic enrollment form is part of the Clinic Tools module in PhilaVax. You will not be able to access the enrollment form until you complete this training.
If you have not completed the Clinic Tools training, sign up now:Â https://vaccines.phila.gov/index.php/notices/register-for-a-required-clinic-tools-training/
If you have completed the Clinic Tools training, check to make sure the contact and staff information for your site is up-to-date. Submit any updates to our program through the module.
- Renew your PhilaVax User Confidentiality Agreement
You must log into the PhilaVax IIS to access and complete the electronic form. If you have not completed the 2018 user confidentiality agreement renewal process or do not have a PhilaVax user account, use the link below to complete it now.
- Make sure your siteâs Medical Director has a PhilaVax Account
Your medical director needs an active PhilaVax account in order to sign the enrollment form. You will not be able to submit the enrollment form without the medical directorâs signature.
Click here to complete a PhilaVax User Confidentiality Agreement: https://docs.google.com/forms/d/1nQ6Kp4WrlVEsx0Ud1QrxCdYRiYP3Rpd70xE0E0RKKscIf you have trouble accessing the above link or your PhilaVax account and need to update your password, you can email PhilaVax@phila.gov or call 215-685-6784.
ÂAdditional information and instructions will be communicated closer to the start of the re-enrollment period on November 1st.
How to defrost your storage unit
Storage units, especially freezers, can sometimes build up too much ice. When you need to defrost your VFC/VFAAR storage unit, TempCheck – our storage and handling coordinators – can help. TempCheck will help you safely storage the vaccine while you defrost the unit.
Use this guide to plan your defrost. Review it when you plan a defrost, and consult it during the defrost process.
If you have any questions, or if your vaccine is exposed to out-of-range temperatures during the defrost or transport process, contact TempCheck as soon as possible.
TempCheck
Adam Howsare and Alexis Bridges
TempCheck@phila.gov
215-685-6777
Plan and prepare
- Contact TempCheck to tell them that you are planning to defrost your unit. TempCheck will need to know when you plan to defrost, and where your back-up unit is. TempCheck will send you a backup DDL to monitor the back-up unit. Keep the packing material so you can send the DDL back to PDPH.
- Prepare the back-up DDL.Ã Once you get the back-up DDL, temper the probe by putting it in your primary freezer. After 30 minutes, start up the DDL. Monitor temperatures for at least 1 hour. Alternatively, place the probe in your unit overnight and start the DDL the next morning.
- Move the vaccine. It’s time to move the vaccine. Leave the primary DDL in your primary unit while you defrost the primary unit.
- If your back-up unit is on-site: Move the vaccine and back-up DDL to your secondary unit. Note the time.
- If your backup unit is off-site: using the emergency transport guide, set up a hard-sided cooler for transport. Move the back-up DDL to the cooler. Place the probe in the middle. Move the vaccine into the cooler. Note the time. Transport the vaccine directly to the back-up location. Note the time the vaccine and DDL are moved into the back-up unit.
Defrosting your unit
- Note the time when you start defrosting your primary unit. The DDL will warm up, which will probably trigger an alarm.
- Allow the ice in the unit to melt.
- Turn the freezer back on. Wait until the DDL shows that the temperature is back in the normal range.
- Transfer the vaccines back into the freezer, using the same transport protocol that you used to move them to the back-up unit.
Dealing with the data
- Download the data from both data loggers and email it to TempCheck@phila.gov.
- Include the following information in the email:
- When you took the vaccine out of the primary unit
- When you put the vaccine into the back-up unit
- When you started defrosting the primary unit
- When you took the vaccine out of the back-up unit
- When you put the vaccine back into the defrosted primary unit
- Reconnect the DDL and continue temperature monitoring as usual
- Repackage the back-up DDL and send it back to PDPH.
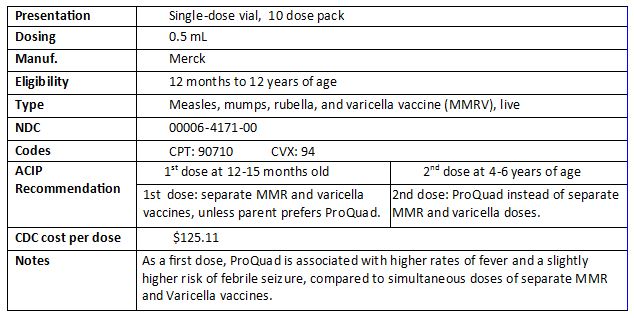
ProQuad now available through VFC
ProQuad vaccine now available through the VFC program
ProQuad is now available through the Philadelphia Vaccines for Children (VFC) program. ProQuad is a combination MMR (measles, mumps, and rubella) and Varicella vaccine. This combined vaccine lets you administer more vaccines with fewer injections.
About ProQuad
The CDCâs Advisory Committee on Immunization Practices recommends ProQuad for a patientâs second dose of MMR and varicella vaccine. As a first dose, ProQuad is associated with a slightly higher risk of febrile seizure, so use separate MMR and varicella vaccines for the first dose unless a parent prefers ProQuad.
- More information: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/mmr.html
- Vaccine Information Statement: http://www.immunize.org/vis/mmrv.pdf
Ordering ProQuad
Order ProQuad through PhilaVax at https://vaccines.phila.gov the way you normally order other vaccines. Before you order ProQuad for your VFC-eligible patients, you should also stock enough privately-purchased ProQuad to treat patients who are not eligible for VFC.
Register for a required Clinic Tools training
Good news! PhilaVax is adding a new tool called Clinic Tools.
Clinic Tools makes it easier to communicate temperature and clinic information with the VFC/VFAAR programs. Through Clinic Tools, you’ll be able to:
- Keep track of storage units and data loggers
- Upload temperatures directly to PhilaVax (no more faxing in paper logs, though you still need to keep them filed for 3 years)
- Maintain up-to-date clinic contact information
- Access and complete your annual VFC/VFAAR enrollment form
Register for a required Clinic Tools training
We need to train you to use this tool. You can attend the training from your own computer. Click here to register. Unable to register? Give us a call at 215-685-6872 (Mohan) or 215-685-6490 (Joani), or email us at DPHProviderHelp@phila.gov and we can help you out.
Training dates
Morning times: 10 AM – 10.30 AM
- December 11
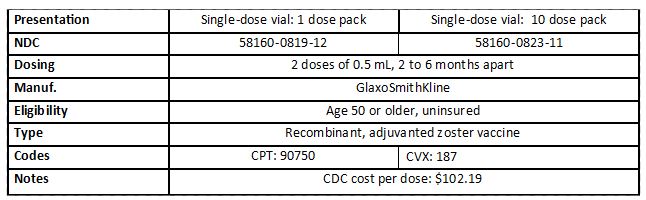
Protect adults from shingles with the new Shingrix vaccine
Protect adults from shingles with the new Shingrix vaccine
Shingrix (recombinant zoster vaccine) is a vaccine to protect healthy adults age 50 and older from shingles. You can now order Shingrix through the Vaccines for Adults At Risk program (VFAAR) for adults 50+ and uninsured.
About Shingrix
Administer Shingrix to adults age 50 and older, with two doses separated by 2 to 6 months.
Ordering Shingrix
Order Shingrix through PhilaVax at https://vaccines.phila.gov the way you normally order other vaccines. We recommend ordering a small amount, initially, to gauge demand. We will work with you to help figure out how much to order: our VFAAR coordinator will review orders and make adjustments to avoid wastage. Before you order Shingrix for your VFAAR-eligible patients, you should also stock enough privately-purchased Shingrix to treat patients who are not eligible for VFAAR.
Storing and Handling
Shingrix must be stored in the refrigerator. Administer it immediately after reconstitution or store it in the refrigerator and use it within 6 hours. Do not freeze Shingrix. If Shingrix freezes, it is spoiled.
Reminder: we need your emergency management plan
The VFC/VFAAR Emergency Vaccine Management Plan has been updated for 2018. This document helps both you and us prepare for an emergency – like if the power goes out or if your refrigerator or freezer stops working.
If you haven’t already done this, we need you to update your information on the Emergency Management Plan and send it to us. To update your plan:
- Download the Emergency Management Plan here
- Print a copy of the plan
- Complete the first page and fax it to us at 215-238-6948
- Gather the supplies needed for emergency transport (listed on pages 6-7) and store them near your storage units
- Post the Emergency Vaccine Management Plan near your storage units
Keep vaccines safe while defrosting – check out our guidance
Freezers can sometimes build up too much ice. When you need to defrost your VFC/VFAAR unit, we can help you move or store your vaccine while you defrost your primary unit. Contact us for defrosting guidance.
