02 Oct 2023 Beyfortus (nirsevimab) Now Available Through VFC
Beyfortus (nirsevimab) Now Available Through VFC
On July 17, 2023, FDA licensed AstraZeneca and Sanofi’s Beyfortus (nirsevimab) for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease (LRTD).
On August 3, the ACIP voted unanimously to recommend nirsevimab for use in infants during the RSV season, which is October through March.
VFC Ordering
Beyfortus (nirsevimab) (5 x 0.5mL syringes) and (5 x 1.0mL syringes) is available to order through the Vaccines for Children (VFC) program starting today, October 2, 2023.
VFC providers who see patients recommended to receive nirsevimab this season are required to include both dosages of nirsevimab in their next VFC order. VFC providers will need to provide nirsevimab for their privately and CHIP insured patients, as applicable.
Dosage and Administration
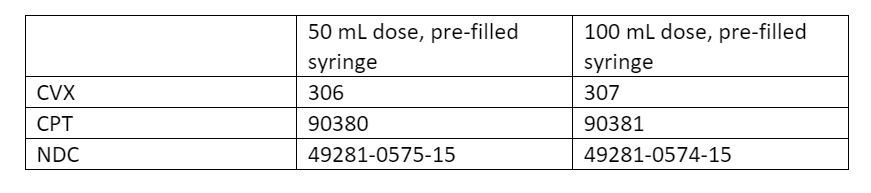
RSV comes in two dosages:
- 50 mg
- 100 mg
Both dosages are administered as an intramuscular injection with a single-dose, pre-filled syringe, and should be ordered to properly administer by weight.
Eligible Patients
- Infants aged <8 months born during or entering their first RSV season
- 1 dose of nirsevimab (50 mg for infants <5 kg and 100 mg for infants ≥5kg)
- Children aged 8-19 months who are at increased risk of severe RSV disease and entering their second RSV season
- 200 mg nirsevimab, administered by two 100 mg syringes
Children at increased risk include those:
- with chronic lung disease resulting from premature birth who required medical support at any time during the six-month period before the start of their second RSV season
- who are severely immune compromised
- with cystic fibrosis who have severe lung disease or whose weight is less than the 10th percentile compared with other babies of the same length
- who are American Indian and Alaska Native
Reporting
All doses of nirsevimab administered in Philadelphia must be reported to PhilaVax.
Storage and Handling
Store refrigerated between 36°F to 46°F (2°C to 8°C) through the expiration date.
Nirsevimab may be kept at room temperature 68°F to 77°F (20°C to 25°C) for a maximum of 8 hours. After removal from the refrigerator, nirsevimab must be used within 8 hours or discarded.
Reporting Adverse Events
Nirsevimab is the first drug product to be included in the VFC program. If nirsevimab is administered:
- alone, suspected adverse events are reported to MedWatch
- simultaneously with any vaccine, suspected adverse events are reported to the Vaccine Adverse Event Reporting System (VAERS); additional reporting to MedWatch not needed
Additional resources:
MMWR
FDA page
Immunization Information Sheet for Patients
Package insert
If you have any questions, please email victor.obeck@phila.gov.


