25 Sep 2025 Enflonsia (Clesrovimab) Now Available Through VFC
On June 9, 2025, the Food and Drug Administration (FDA) licensed Merck’s Enflonsia (clesrovimab) for the prevention of respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI).
On June 26, 2025, the Advisory Committee on Immunization Practices
(ACIP) voted to recommend clesrovimab for use in infants younger than 8 months of age during RSV season, which is October through March.
VFC Ordering
Enflonsia (clesrovimab) (105 mg/0.7 mL syringes) will be available to order through the Vaccines for Children (VFC) program starting October 2, 2025. It will be available in one- and 10-dose packaging.
To add this formulation to your office’s list of vaccines, use the button below to complete a change request form. Providers interested in ordering clesrovimab on October 2 should complete the form by Friday, September 26, at 12 p.m.
Our team will review your request and reply with next steps. We recommend that providers that are affiliated or part of a system use the same vaccine presentations across sites to ensure standardization and help prevent administration errors. Please note that providers who add clesrovimab for the 2025-2026 season may need to order both nirsevimab and clesrovimab due to clesrovimab supply limitations.
Dosage and Administration
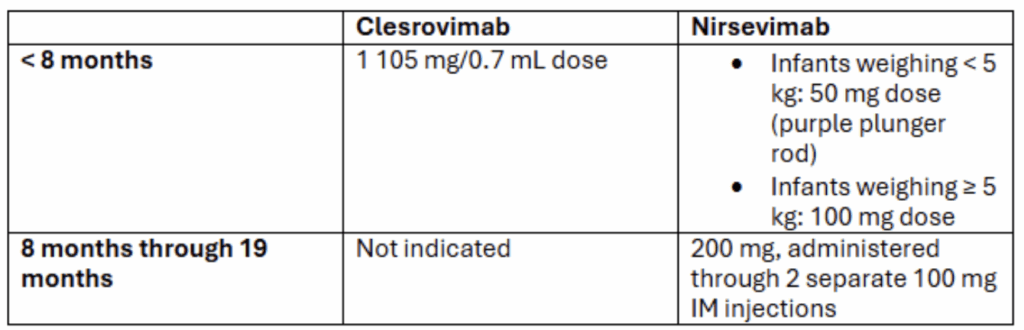
Clesrovimab is available in one dosage, 105 mg/0.7 mL, administered as an intramuscular injection with a single-dose, pre-filled syringe.
Monoclonal Antibodies Available for Infant Vaccinations
Clesrovimab is the second monoclonal antibody to be approved for use for the prevention of RSV LRTI in infants.
- Clesrovimab or nirsevimab can be given to infants younger than 8 months of age born during or entering their first RSV season
- No preferential recommendation for use of clesrovimab versus nirsevimab
- Only nirsevimab is recommended for children ages 8 through 19 months who are at increased risk of severe RSV disease and entering their second RSV season
- Infants eligible to receive nirsevimab when entering their second RSV season can receive nirsevimab or clesrovimab for their first RSV season
RSV Immunization Product Indications
Eligible Patients
- Infants aged < 8 months born during or entering their first RSV season
- 1 dose of clesrovimab
Reporting
All doses of clesrovimab administered in Philadelphia must be reported to PhilaVax.
Clesrovimab Product Information

Storage and Handling
Store refrigerated between 36°F to 46°F (2°C to 8°C) through the expiration date.
Clesrovimab may be kept at room temperature – 68°F to 77°F (20°C to 25°C) – for a maximum of 48 hours. After removal from the refrigerator, clesrovimab must be used within 48 hours or discarded.
Reporting Adverse Events
If clesrovimab is administered:
- alone, suspected adverse events are reported to MedWatch
- simultaneously with any vaccine, suspected adverse events are reported to the Vaccine Adverse Event Reporting System (VAERS); additional reporting to MedWatch not needed
Additional Resources
If you have any questions, please email DPHProviderHelp@phila.gov.


