14 Dec 2022 Moderna & Pfizer Shelf-Life Extensions
Posted at 12:29h
in COVID-19
Moderna & Pfizer Shelf-Life Extensions
FDA has approved additional Moderna shelf-life extensions. With these extensions, there are now Moderna and Pfizer-BioNTech monovalent COVID-19 vaccines that will last into spring 2023. As a matter of best practice, please verify expiry dates using the manufacturers’ online checkers prior to vaccine disposal.
MODERNA
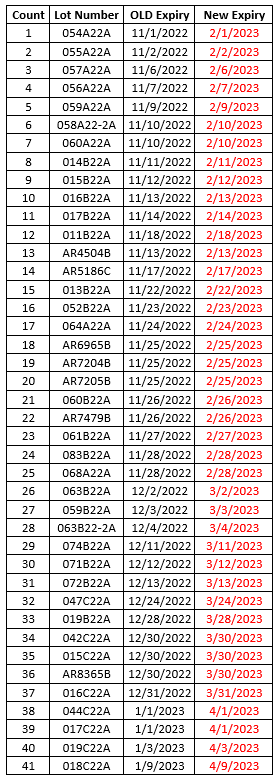
- Moderna has now received shelf-life extensions of all wave 1 and wave 2 monovalent COVID-19 vaccines (complete list below).
- Some of the lots below are MOD 10 (ages 12+) and some are MOD 5 (ages 6-11). All Moderna lots that have received shelf-life extensions are for primary series use only.
- Please use the Moderna Vial Expiration Checker to check expiration dates.
PFIZER
- Pfizer has received shelf-life extensions of all TRIS products (i.e., all mRNA COVID-19 vaccines, including both monovalent and bivalent vaccines).
- Expiration is now 18 months from the date of manufacture (stored ULT frozen).
- Please use the Pfizer-BioNTech COVID-19 Vaccine Expiry tool to check expiration dates.
REMINDERWe continue to encourage you to properly manage and report your inventory:
- Practice first in/first out inventory management.
- Store older vaccines to the front of the refrigerator or freezer unit.
- Continually monitor vaccines to help reduce administration errors.
- Verify expiry dates using the manufacturers’ online checkers:
- Pfizer: https://lotexpiry.cvdvaccine.com
- Moderna: https://modernacovid19global.com/vial-lookup
- Novavax: https://us.novavaxcovidvaccine.com/hcp
- Johnson and Johnson/Janssen: https://vaxcheck.jnj/
- Dispose of any expired vaccine according to state and local regulations.
- Update/zero out inventory in VaccineFinder to avoid turning away customers.CDC’s COVID-19 Vaccine Lot Number and Expiration Date Report: https://vaccinecodeset.cdc.gov/LotNumber


