20 Jul 2021 Did Your Patient Receive a COVID-19 Vaccination Outside the United States?
Did Your Patient Receive a COVID-19 Vaccination Outside the United States?
Have questions? The “People vaccinated outside the United States” section of the CDC’s Interim Clinical Considerations for Use of COVID-19 Vaccines provides guidance on different scenarios.
Talk to your patient and gather the initial information:
- Which COVID-19 vaccine did the patient receive?
- Is the COVID-19 vaccine authorized by the United States Food and Drug Administration (FDA) or listed for emergency use by the World Health Organization (WHO)?
- Did your patient complete a full series of this COVID-19 vaccine (e.g. receive both doses if two doses are recommended)?
Guidelines as of 7/15/21:
US FDA-authorized COVID-19 vaccines:
- Pfizer-BioNTech (Comirnaty, Tozinameran; mRNA nucleoside modified) – 2 doses
- Moderna (Spikevax; mRNA nucleoside modified) – 2 doses
- Johnson & Johnson / Janssen (Ad26.COV2-S recombinant) – 1 dose
COVID-19 vaccines listed for emergency use by the WHO:
- US FDA-authorized vaccines above
- AstraZeneca-Oxford (Covishield, Vaxzevria; ChAdOx1-S recombinant) – 2 doses
- Sinopharm (Vero Cell inactivated) – 2 doses
- Sinovac (Vero Cell inactivated) – 2 doses
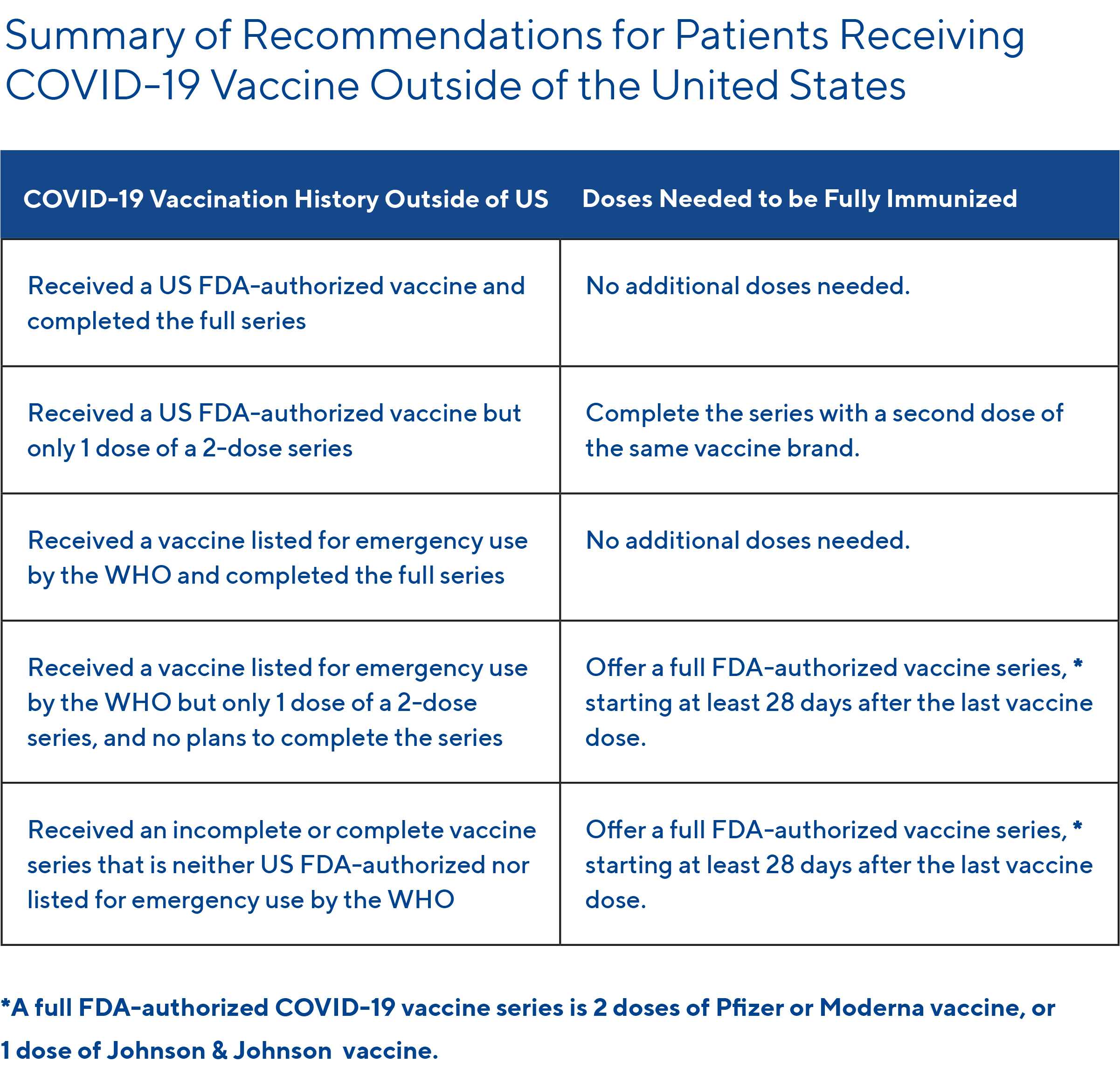
See the table below for a summary of recommendations for patients who received a COVID-19 vaccination outside of the US:
Resources:
- WHO – Prequalification of Medical Products: Coronavirus Disease
- Regulation and Prequalification: COVID-19 vaccines
- CDC’s Interim Clinical Considerations for Use of COVID-19 Vaccines
- New York Times: COVID World Vaccination Tracker Map
Thank you for keeping Philadelphia safe and healthy! If you have any questions, please email vaccines@phila.gov.

