New Immigrant and Refugee Pediatric Vaccination
This blog post was written alongside Dr. Mayssa Abuali, who has served the Philadelphia pediatric community for the past 10 years.
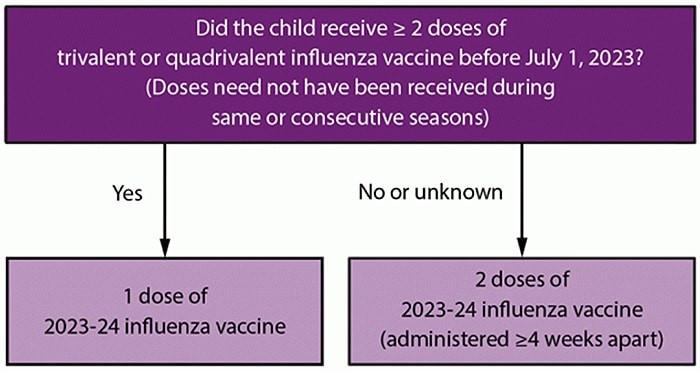
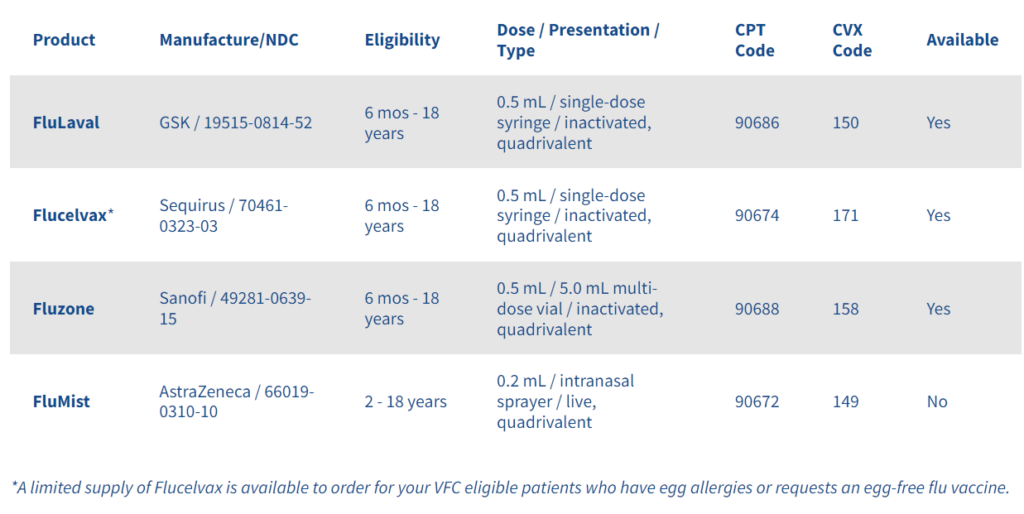
At the first domestic health visit with new immigrant or refugee pediatric patients, clinicians should review all available vaccine records, perform any testing, and update or revaccinate, as appropriate. Vaccine doses administered outside the United States should be accepted as valid, if schedules and doses are compatible with the Advisory Committee in Immunization Practices (ACIP) recommendations. If there’s no proof of receiving the required vaccines, it must be given during the medical exam.
Catch-up vaccines:
- Work closely with Philadelphia School District nurses to catch up a student’s vaccines. This ensures timely school enrollment and protection of other students from vaccine preventable diseases.
- Pay careful attention to intervals between vaccines as well as minimum required ages, and not only the number of doses previously received.
- Attach any relevant serology to the child’s health assessment form or vaccine records. For example, include varicella and hepatitis A IgG titers and indicate if the child is immune by serology or natural disease.
Polio vaccination: In April 2016, the oral polio vaccine (OPV) was changed from a trivalent to a bivalent formulation to decrease the risk of vaccine-associated paralytic disease. Any oral polio vaccine given after April 2016 is invalid in the United States and will not count towards the child’s required polio series. Inactivated polio injectable vaccine (IPV) doses are needed to replace invalid OPV doses.
Transcribing vaccines: Many countries place the day before the month (i.e. day/month/year). Train your team to remember such date formats when transcribing international vaccine records into EMR.
Schedule follow-ups: Provide a scheduled follow-up visit for the vaccine before the patient leaves the clinic and indicate “catch-up vaccination initiated; follow-up scheduled on _” on the child health assessment form.