Monkeypox Vaccine Update: Expanded Eligibility & Second Doses
Monkeypox Vaccine Update: Expanded Eligibility & Second Doses
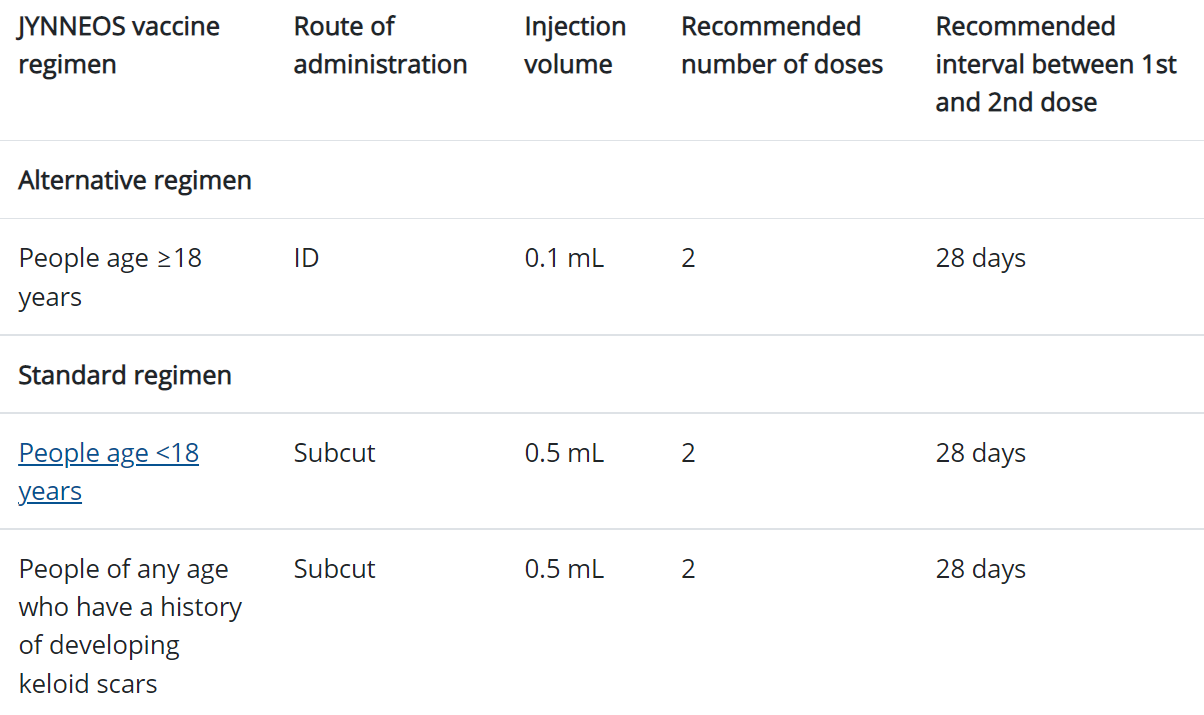
There is now vaccine supply to begin administering a complete two-dose Jynneos vaccine series to each individual who is eligible for vaccine.
Updates
Eligibility for a first dose now includes:
• People of all ages. Subcutaneous administration continues to be required for those <18.
• People with a history of STI in the past 12 months, as well as individuals who plan to have multiple or anonymous sex partners, or those plan to meet new sexual partners via social media.
Eligibility for a second dose now includes:
• People who have received a first Jynneos vaccine dose through either intradermal or subcutaneous administration. This dose should be given intradermally to those 18 and older unless there is a contraindication.
Eligibility has also expanded to include those with a history of STI in the past 12 months (from 6 months previously) and to start offering preexposure prophylaxis to people at high risk for becoming infected. The interval between doses for both subcutaneous and intradermal injections is at minimum 28 days. Individuals who test positive for monkeypox after their first dose should not receive a second dose of vaccine. Individuals whose second doses have been delayed do not need to restart the two-dose vaccine series.
There is no age requirement for Jynneos vaccine eligibility, although those less than 18 years of age must receive the vaccine subcutaneously. Individuals 18 and older will be given intradermal vaccination, regardless of which method was used for the first vaccine, unless there is a contraindication.
Complete eligibility criteria include:
People who meet the following condition:
• Gay, bisexual, transgender, non-binary, and other men who have sex with men, transgender, or non-binary persons
AND meet ONE of the following criteria:
• Have had multiple or anonymous sex partners in the last 14 days
• Have had any newly diagnosed STI in the past 12 months, including gonorrhea, chlamydia, early syphilis, or HIV
• Have recently attended or plan to attend any venue where anonymous sex or sex with multiple partners will occur (e.g. saunas, bathhouses, sex clubs, sex parties) in the next 30 days
• Have met recent partners or plan to meet new partners in the next 30 days through social media platforms (such as Grindr, Tinder or Scruff), or at clubs, raves, sex parties, saunas
Additionally, the following people are eligible:
• Sex workers (of any sex or gender), and/or
• Anyone with known close contact (skin-to-skin) with someone with monkeypox in the past 14 days
When using an intradermal strategy, there may be unused doses in a single vial. All efforts should be made to give doses to the above eligible individuals. If there are doses that would go wasted, they may be given to other individuals not included in the above criteria. Healthcare workers directly involved in testing and examining those with possible monkeypox illness and staff involved in environmental cleaning and disinfection protocols should be prioritized.
Thank you for keeping Philadelphia safe and healthy! If you have any questions about these updates, please contact vaccines@phila.gov.