Menu
Contact usProvider? Stay in the know
Get the newest information from the Immunization Program right in your inbox!
Sign upProper vaccine storage and handling are critical in preventing vaccine-preventable diseases. This page provides guidance on setting up and maintaining proper vaccine storage units at your site.
215-685-6777
To protect the viability of the federally funded vaccines stored at your site, they must be stored in the acceptable temperature ranges indicated below.
To maintain proper vaccine temperature, ensure the following are in place at your site:
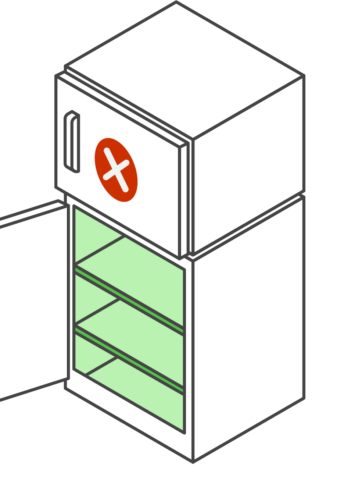
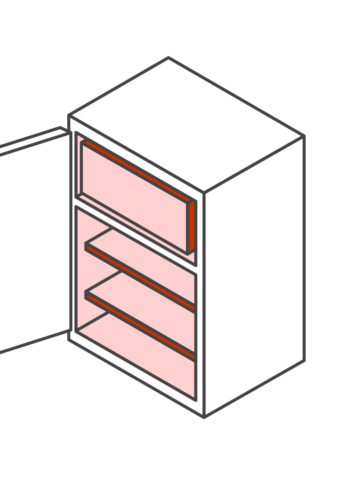
Your vaccine storage unit is a key component in maintaining the vaccine cold chain at your practice. See below for an overview of storage unit requirements.
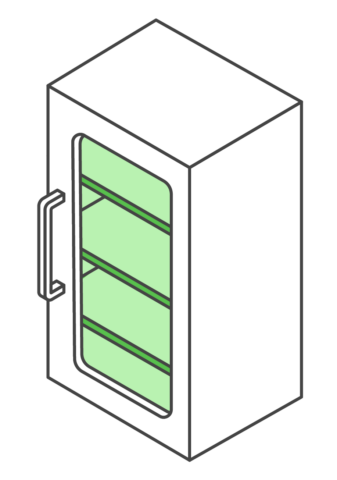
Your storage unit must:
Your storage unit must not:
Email tempcheck@phila.gov for help with selecting the unit that’s best for your practice.
Follow these storage steps to protect your vaccine viability and prevent wastage.
Before you can store any federally funded vaccine in your storage unit, it must be approved by the Immunization Program. Follow the steps below to get started.
Send pictures of the following for each prospective storage unit to tempcheck@phila.gov:


Storage units need regular maintenance to ensure proper operation. Conduct routine maintenance for all vaccine storage units and related equipment – such as generators, alarm systems, and back-up batteries – so that your equipment functions at maximum efficiency:
Vaccine transport refers to any time vaccine needs to be moved from primary units at the site to another location.
The requirement for continuous temperature monitoring with a DDL extends to transport. Whenever the vaccine needs to be moved, a DDL needs to be with the vaccine at all times.
This can mean requesting a back-up DDL from the Immunization Program or moving the DDL from your primary unit with the vaccine.
Vaccine can only be moved from an approved and monitored storage unit to another approved and monitored storage unit. If the receiving unit does not have at least 48 hours of temperatures, then that unit is not ready to receive vaccines.
Federally funded vaccine cannot be transported in a non-emergency situation without prior permission from the Immunization Program.
Non-emergency situations include:
Units that have been moved – from a location within the office or a location outside the office – may not be able to maintain temperatures in its new location for a variety of reasons. These include:
Unit must be re-approved when moved to a new location.
Contact tempcheck@phila.gov for further guidance.