Menu
Contact usProvider? Stay in the know
Get the newest information from the Immunization Program right in your inbox!
Sign upIt is important to be prepared and act immediately in case a storage unit’s temperature goes out of range. Vaccines that are subject to out-of-range temperatures may lose potency and no longer be effective.
Download and print the vaccine management plan so you have access to crucial information at all times.
215-685-6777
Every provider is required to identify two (2) backup locations where they can move their vaccine in the event of power outages and equipment malfunctions. Both of these locations must be off-site from your location.
These locations must be large enough for your location’s typical supply of vaccine and meet the temperature monitoring requirements of the Immunization program.
The address and contact information for the back-up locations should be recorded in your vaccine management plan.
If you cannot identify two locations, please reach out to tempcheck@phila.gov.
Approved locations
Unapproved locations
Identify a non-office hours contact who can access to the vaccine during non-clinic hours.
Record this person’s information in the vaccine management plan.
Ideally, vaccine should be transported in a portable vaccine refrigerator or a qualified pack out.
Alternatively, gather the supplies below ahead of time so you can respond quickly and keep your vaccine within acceptable temperature ranges during transport.
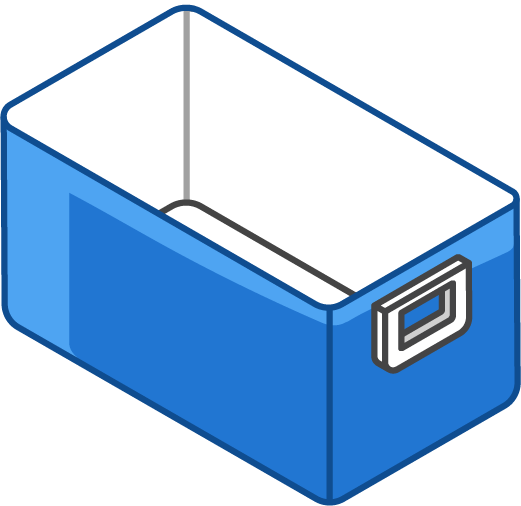
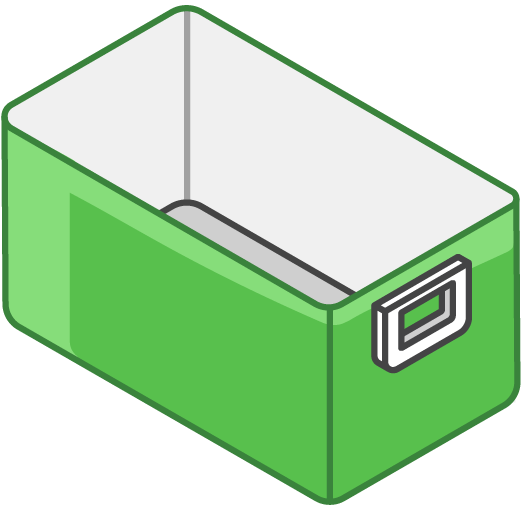
Hard-sided cooler(s):

Cushioning material:

Frozen water bottles:

Corrugated cardboard:
Close the lid
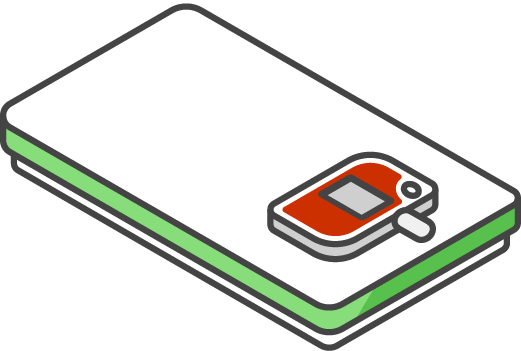
Attach the DDL display and your paper temperature log to the top of the lid

Fill the remaining space in the cooler
Cut to fit the dimensions of your cooler to support the water bottles
Cover vaccine with another 1” thick layer of bubble wrap, packing foam, or Styrofoam™
Stack boxes of vaccine and dilutents
When cooler is halfway full, place the buffered probe in the center of vaccine
Cover the probe with remaining vaccine
At least 1” thick layer of bubble wrap, packing foam, or Styrofoam™

Cut to fit the dimensions of your hard-sided-cooler

Line bottom of cooler with a single layer of conditioned frozen water bottles

Large enough for your typical supply of refrigerated vaccine
Can use original shipping boxes from manufacturers if available
Close the lid
Attach the DDL display and your paper temperature log to the top of the lid
Layer at least two (2) inches thick and cover all the remaining empty space

Place a layer that completely covers the vaccines

Stack vaccine directly on top of frozen water bottles below
Place the buffered temperature probe with the top layer of vaccines

Line bottom of cooler with a single layer of frozen water bottles
Never use dry ice

Large enough for your typical supply of frozen vaccine
Can use original shipping boxes from manufacturers if available
![]()
For refrigerated vaccine transport:
Frozen water bottles must be conditioned before use to transport refrigerated vaccine.
To condition: Submerge in lukewarm water or hold under running water until the ice spins freely inside the bottle, then dry the outside of the bottle.
For frozen vaccine transport:
Do not condition frozen water bottles to transport frozen vaccine.
![]()
Transport the diluent the same way that you store it normally:
Upon arrival at the back-up location, document the following:
Monitor temperature of the storage unit twice a day on your paper temperature logs.
Contact tempcheck@phila.gov (215-685-6777) to plan to transport the vaccine back to your site once the original issue is resolved.
![]()
Notify us at tempcheck@phila.gov (215-685-6777) if there is an out-of-range temperature or you need to transport the vaccines.
![]()
Keep your DDL with the vaccine at all times, including transport.
![]()
Keep a record of all actions you take during the event and how long they take.
![]()
Notify key staff such as the Vaccine Coordinator or backup, and the Medical Director or Chief Medical Officer.
![]()
Do not manipulate the digital data logger (DDL) sensor probe.
Cooling or warming the probe does not accurately reflect the temperature of the vaccines. Manipulating the probe can lead to the administration of non-viable vaccine to patients.
Different types of emergencies warrant different responses. Find the section below that aligns with your situation and follow the listed procedures.
These all assume your vaccine have not yet experienced out-of-range temperatures. Guidance on temperature excursions can be found lower on this page.
Continue to use vaccines that have stayed in the proper temperature range.
If any of your vaccine has experienced an out-of-range temperature, follow the guidance listed below on this page about what to do after an excursion.
A temperature excursion is any time your vaccine is exposed to an out-of-range temperature. This puts your vaccine at risk of losing potency and becoming ineffective.
Your DDL will alert you when your unit is experiencing a temperature excursion.
Whether you hear the alarm or notice an out-of-range temperature yourself, it is crucial to act immediately and follow the guidance below.
![]()
Contact tempcheck@phila.gov (215-685-6777)
![]()
Consult your vaccine management plan
![]()
For vaccine that has experienced an out-of-range temperature:
![]()
Notify your Vaccine Coordinator or backup coordinator
![]()
Record the out-of-range temperatures and the room temperature in the bottom section of your paper temperature logs
![]()
Do not discard vaccine unless directed by the Philadelphia Immunization Program
For some storage unit excursions, the Immunization Program will require that you contact the vaccine manufacturers and complete an Excursion Response Form.
This information must come directly from the manufacturers. Sites are required to provide a case number for each manufacturer.
Immunization Program staff will audit these forms. A new form is required every time you report an excursion event.
Do not assume viability of vaccines. Always contact the manufacturer.
Providers are accountable for any vaccine supplied by the VFC/VFAAR programs and can be held responsible for reimbursement of spoiled vaccine due to negligence.
This reimbursement will consist of dose-for-dose vaccine replacement at the private cost.
Contact DPHProviderHelp@phila.gov for more information.
Vaccine that has expired, spoiled, or been wasted must be reported through PhilaVax immediately. Orders for additional vaccine may be delayed if this is not completed.
For more guidance on completing a wastage or return, click here.